Complexation of Chol-DsiRNA in place of Chol-siRNA greatly increases the duration of mRNA suppression by polyplexes of PLL(30)-PEG(5K) in primary murine syngeneic breast tumors after i.v. administration
- PMID: 29601972
- PMCID: PMC5927825
- DOI: 10.1016/j.ijpharm.2018.03.045
Complexation of Chol-DsiRNA in place of Chol-siRNA greatly increases the duration of mRNA suppression by polyplexes of PLL(30)-PEG(5K) in primary murine syngeneic breast tumors after i.v. administration
Abstract
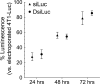
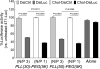
RNA interference has tremendous potential for cancer therapy but is limited by the insufficient potency of RNAi molecules after i.v. administration. We previously found that complexation with PLL(30)-PEG(5K) greatly increases the potency of 3'-cholesterol-modified siRNA [Chol-siRNA] in primary murine syngeneic 4T1 breast tumors after i.v. administration but mRNA suppression decreases 24 h after the final dose. We hypothesized that complexation of cholesterol-modified Dicer-substrate siRNA (Chol-DsiRNA) in place of Chol-siRNA can increase the potency and duration of suppression by polyplexes of PLL(30)-PEG(5K) in solid tumors. We found that replacing Chol-siRNA with Chol-DsiRNA increased polyplex loading and nuclease protection, suppressed stably expressed luciferase to the same extent in primary murine 4T1-Luc breast tumors under the current dosage regimen, but maintained suppression ~72 h after the final dose. The kinetics of suppression in 4T1-Luc over 72 h, however, were similar between DsiLuc and siLuc after electroporation and between polyplexes of Chol-DsiLuc and Chol-siLuc after transfection, suggesting that Chol-DsiRNA polyplexes increase the duration of mRNA suppression through differences in polyplex activities in vivo. Thus, replacing Chol-siRNA with Chol-DsiRNA may significantly increase the duration of mRNA suppression by polyplexes of PLL(30)-PEG(5K) and possibly other PEGylated polycationic polymers in primary tumors and metastases after i.v. administration.
Keywords: Chol-DsiRNA polymer micelles; Chol-DsiRNA polyplexes; Chol-siRNA polymer micelles; Chol-siRNA polyplexes; Drug delivery; DsiRNA; RNA interference.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures


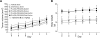

References
-
- Aigner A. Cellular delivery in vivo of siRNA-based therapeutics. Curr Pharm Des. 2008;14:3603–3619. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

