Regenerative abilities of mesenchymal stem cells through mitochondrial transfer
- PMID: 29602309
- PMCID: PMC5877369
- DOI: 10.1186/s12929-018-0429-1
Regenerative abilities of mesenchymal stem cells through mitochondrial transfer
Abstract
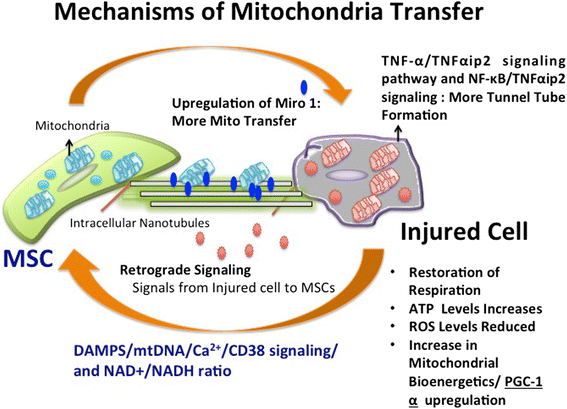
The past decade has witnessed an upsurge in studies demonstrating mitochondrial transfer as one of the emerging mechanisms through which mesenchymal stem cells (MSCs) can regenerate and repair damaged cells or tissues. It has been found to play a critical role in healing several diseases related to brain injury, cardiac myopathies, muscle sepsis, lung disorders and acute respiratory disorders. Several studies have shown that various mechanisms are involved in mitochondrial transfer that includes tunnel tube formation, micro vesicle formation, gap junctions, cell fusion and others modes of transfer. Few studies have investigated the mechanisms that contribute to mitochondrial transfer, primarily comprising of signaling pathways involved in tunnel tube formation that facilitates tunnel tube formation for movement of mitochondria from one cell to another. Various stress signals such as release of damaged mitochondria, mtDNA and mitochondrial products along with elevated reactive oxygen species levels trigger the transfer of mitochondria from MSCs to recipient cells. However, extensive cell signaling pathways that lead to mitochondrial transfer from healthy cells are still under investigation and the changes that contribute to restoration of mitochondrial bioenergetics in recipient cells remain largely elusive. In this review, we have discussed the phenomenon of mitochondrial transfer from MSCs to neighboring stressed cells, and how this aids in cellular repair and regeneration of different organs such as lung, heart, eye, brain and kidney. The potential scope of mitochondrial transfer in providing novel therapeutic strategies for treatment of various pathophysiological conditions has also been discussed.
Keywords: Mesenchymal stem cells; Mitochondrial transfer mechanism; Regenerative medicine.
Conflict of interest statement
Ethics approval and consent to participate
This study was ethically approved by the Institutional Committee for Stem Cell Research (IC-SCR), All India Institute of Medical Sciences (AIIMS), New Delhi.
Consent for publication
All authors read the final manuscript and approved manuscript for publication. Table 1 represents data from existing literature. Written informed consent was obtained from the patient for accompanying images (Figs. 1 and 4) in this review. The consent form is held by the authors/by the authors’ institution and is available for review by the Editor-in-Chief.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage.Cell Death Dis. 2016 Nov 10;7(11):e2467. doi: 10.1038/cddis.2016.358. Cell Death Dis. 2016. PMID: 27831562 Free PMC article.
-
Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung.Stem Cell Res Ther. 2016 Jul 12;7(1):91. doi: 10.1186/s13287-016-0354-8. Stem Cell Res Ther. 2016. PMID: 27406134 Free PMC article.
-
Horizontal mitochondrial transfer as a novel bioenergetic tool for mesenchymal stromal/stem cells: molecular mechanisms and therapeutic potential in a variety of diseases.J Transl Med. 2024 May 24;22(1):491. doi: 10.1186/s12967-024-05047-4. J Transl Med. 2024. PMID: 38790026 Free PMC article. Review.
-
Mitochondrial transfer from Wharton's jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function.Mitochondrion. 2015 May;22:31-44. doi: 10.1016/j.mito.2015.02.006. Epub 2015 Mar 3. Mitochondrion. 2015. PMID: 25746175
-
Mechanisms behind therapeutic potentials of mesenchymal stem cell mitochondria transfer/delivery.J Control Release. 2023 Feb;354:755-769. doi: 10.1016/j.jconrel.2023.01.059. Epub 2023 Jan 27. J Control Release. 2023. PMID: 36706838 Review.
Cited by
-
The effects of BMMSC treatment on lung tissue degeneration in elderly macaques.Stem Cell Res Ther. 2021 Mar 1;12(1):156. doi: 10.1186/s13287-021-02201-3. Stem Cell Res Ther. 2021. PMID: 33648583 Free PMC article.
-
Targeting mitochondrial quality control of T cells: Regulating the immune response in HCC.Front Oncol. 2022 Sep 23;12:993437. doi: 10.3389/fonc.2022.993437. eCollection 2022. Front Oncol. 2022. PMID: 36212470 Free PMC article. Review.
-
Mesenchymal Stem Cell-Based Therapy for Retinal Degenerative Diseases: Experimental Models and Clinical Trials.Cells. 2021 Mar 7;10(3):588. doi: 10.3390/cells10030588. Cells. 2021. PMID: 33799995 Free PMC article. Review.
-
Cellular In Vitro Responses Induced by Human Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles Obtained from Suspension Culture.Int J Mol Sci. 2024 Jul 11;25(14):7605. doi: 10.3390/ijms25147605. Int J Mol Sci. 2024. PMID: 39062847 Free PMC article.
-
Therapeutic Effects of Mesenchymal Stromal Cells Require Mitochondrial Transfer and Quality Control.Int J Mol Sci. 2023 Oct 31;24(21):15788. doi: 10.3390/ijms242115788. Int J Mol Sci. 2023. PMID: 37958771 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

