Metabolomics and Metabolic Reprogramming in Kidney Cancer
- PMID: 29602399
- PMCID: PMC6009840
- DOI: 10.1016/j.semnephrol.2018.01.006
Metabolomics and Metabolic Reprogramming in Kidney Cancer
Abstract
Kidney cancer, or renal cell carcinoma (RCC), is a disease of increasing incidence that commonly is seen in the general practice of nephrology. Despite this state of affairs, this fascinating and highly morbid disease frequently is under-represented, or even absent, from the curriculum of nephrologists in training and generally is underemphasized in national nephrology meetings, both scientific as well as clinical. Although classic concepts in cancer research in general had led to the concept that cancer is a disease resulting from mutations in the control of growth-regulating pathways, reinforced by the discovery of oncogenes, more contemporary research, particularly in kidney cancer, has uncovered changes in metabolic pathways mediated by those same genes that control tumor energetics and biosynthesis. This adaptation of classic biochemical pathways to the tumor's advantage has been labeled metabolic reprogramming. For example, in the case of kidney cancer there exists a near-universal presence of von Hippel-Lindau tumor suppressor (pVHL) inactivation in the most common form, clear cell RCC (ccRCC), leading to activation of hypoxia-relevant and other metabolic pathways. Studies of this and other pathways in clear cell RCC (ccRCC) have been particularly revealing, leading to the concept that ccRCC can itself be considered a metabolic disease. For this reason, the relatively new method of metabolomics has become a useful technique in the study of ccRCC to tease out those pathways that have been reprogrammed by the tumor to its maximum survival advantage. Furthermore, identification of the nodes of such pathways can lead to novel areas for drug intervention in a disease for which such targets are seriously lacking. Further research and dissemination of these concepts, likely using omics techniques, will lead to clinical trials of therapeutics specifically targeted to tumor metabolism, rather than those generally toxic to all proliferating cells. Such novel agents are highly likely to be more effective than existing drugs and to have far fewer adverse effects. This review provides a general overview of the technique of metabolomics and then discusses how it and other omics techniques have been used to further our understanding of the basic biology of kidney cancer as well as to identify new therapeutic approaches.
Keywords: Kidney cancer; metabolomics; reprogramming; therapeutics.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
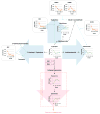
References
-
- Warburg O. On the origin of cancer cells. Science (New York, NY) 1956;123:309–14. - PubMed
-
- Weiss RH, Lin P-Y. Kidney Cancer: Identification of Novel Targets for Therapy. Kidney Int. 2006;69:224–32. - PubMed
-
- Wettersten HI, Aboud OA, Lara PN, Jr, Weiss RH. Metabolic reprogramming in clear cell renal cell carcinoma. Nature reviews Nephrology. 2017 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

