Formation of Both Heme and Apoprotein Adducts Contributes to the Mechanism-Based Inactivation of Human CYP2J2 by 17 α-Ethynylestradiol
- PMID: 29602797
- PMCID: PMC5931439
- DOI: 10.1124/dmd.118.080903
Formation of Both Heme and Apoprotein Adducts Contributes to the Mechanism-Based Inactivation of Human CYP2J2 by 17 α-Ethynylestradiol
Abstract
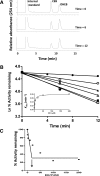
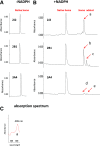
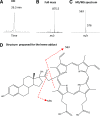
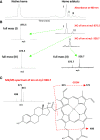
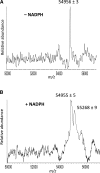
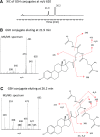
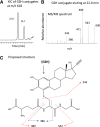
17α-Ethynylestradiol (EE), a major component of many oral contraceptives, affects the activities of a number of the human cytochrome P450 (P450) enzymes. Here, we characterized the effect of EE on CYP2J2, a major human P450 isoform that participates in metabolism of arachidonic acid. EE inactivated the hydroxyebastine carboxylation activity of CYP2J2 in a reconstituted system. The loss of activity is time and concentration dependent and requires NADPH. The KI and kinact values for the inactivation were 3.6 μM and 0.08 minute-1, respectively. Inactivation of CYP2J2 by EE was due to formation of a heme adduct as well as an apoprotein adduct. Mass spectral analysis of CYP2J2 partially inactivated by EE showed two distinct protein masses in the deconvoluted spectrum that exhibited a mass difference of approximately 312 Da, which is equivalent to the sum of the mass of EE and one oxygen atom. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis revealed a heme adduct with MH+ ion at m/z 875.5, corresponding to alkylation of an iron-depleted prosthetic heme by EE plus one oxygen atom. The reactive intermediate responsible for covalently modifying both the prosthetic heme and apoprotein was characterized by trapping with glutathione (GSH). LC-MS/MS analysis revealed two GSH conjugate isomers with MH+ ions at m/z 620, which were formed by reaction between GSH and EE with the oxygen being added to either the internal or terminal carbon of the ethynyl moiety. High-pressure liquid chromatography analysis revealed that three other major metabolites were formed during EE metabolism by CYP2J2.
Copyright © 2018 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
References
-
- Ahluwalia BS, Curry CL, Crocker CL, Verma PS. (1977) Evidence of higher ethynylestradiol blood levels in human hypertensive oral contraceptive users. Fertil Steril 28:627–630. - PubMed
-
- Baillie TA, Davis MR. (1993) Mass spectrometry in the analysis of glutathione conjugates. Biol Mass Spectrom 22:319–325. - PubMed
-
- Bolt HM, Bolt M, Kappus H. (1977) Interaction of rifampicin treatment with pharmacokinetics and metabolism of ethinyloestradiol in man. Acta Endocrinol (Copenh) 85:189–197. - PubMed
-
- Chan WK, Sui Z, Ortiz de Montellano PR. (1993) Determinants of protein modification versus heme alkylation: inactivation of cytochrome P450 1A1 by 1-ethynylpyrene and phenylacetylene. Chem Res Toxicol 6:38–45. - PubMed
-
- Chang SY, Chen C, Yang Z, Rodrigues AD. (2009) Further assessment of 17α-ethinyl estradiol as an inhibitor of different human cytochrome P450 forms in vitro. Drug Metab Dispos 37:1667–1675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

