SMAD4 Gene Mutation Renders Pancreatic Cancer Resistance to Radiotherapy through Promotion of Autophagy
- PMID: 29602802
- PMCID: PMC6345154
- DOI: 10.1158/1078-0432.CCR-17-3435
SMAD4 Gene Mutation Renders Pancreatic Cancer Resistance to Radiotherapy through Promotion of Autophagy
Abstract
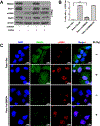
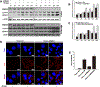
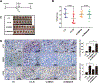
Purpose: Understanding the mechanism of radioresistance could help develop strategies to improve therapeutic response of patients with PDAC. The SMAD4 gene is frequently mutated in pancreatic cancer. In this study, we investigated the role of SMAD4 deficiency in pancreatic cancer cells' response to radiotherapy.Experimental Design: We downregulated SMAD4 expression with SMAD4 siRNA or SMAD4 shRNA and overexpressed SMAD4 in SMAD4 mutant pancreatic cancer cells followed by clonogenic survival assay to evaluate their effects on cell radioresistance. To study the mechanism of radioresistance, the effects of SMAD4 loss on reactive oxygen species (ROS) and autophagy were determined by flow cytometry and immunoblot analysis, respectively. Furthermore, we measured radioresistance by clonogenic survival assay after treatment with autophagy inhibitor (Chloroquine) and ROS inhibitor (N-acetyl-l-cysteine) in SMAD4-depleted pancreatic cancer cells. Finally, the effects of SMAD4 on radioresistance were also confirmed in an orthotopic tumor model derived from SMAD4-depleted Panc-1 cells.Results:SMAD4-depleted pancreatic cancer cells were more resistant to radiotherapy based on clonogenic survival assay. Overexpression of wild-type SMAD4 in SMAD4-mutant cells rescued their radiosensitivity. Radioresistance mediated by SMAD4 depletion was associated with persistently higher levels of ROS and radiation-induced autophagy. Finally, SMAD4 depletion induced in vivo radioresistance in Panc-1-derived orthotopic tumor model (P = 0.038). More interestingly, we observed that the protein level of SMAD4 is inversely correlated with autophagy in orthotopic tumor tissue samples.Conclusions: Our results demonstrate that defective SMAD4 is responsible for radioresistance in pancreatic cancer through induction of ROS and increased level of radiation-induced autophagy. Clin Cancer Res; 24(13); 3176-85. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
There are no financial disclosures or conflicts of interest to report for any of the authors.
Figures
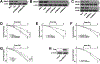
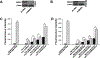
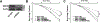
Similar articles
-
SMAD4 Limits PARP1 dependent DNA Repair to Render Pancreatic Cancer Cells Sensitive to Radiotherapy.Cell Death Dis. 2024 Nov 11;15(11):818. doi: 10.1038/s41419-024-07210-7. Cell Death Dis. 2024. PMID: 39528473 Free PMC article.
-
MicroRNA 23b regulates autophagy associated with radioresistance of pancreatic cancer cells.Gastroenterology. 2013 Nov;145(5):1133-1143.e12. doi: 10.1053/j.gastro.2013.07.048. Epub 2013 Aug 2. Gastroenterology. 2013. PMID: 23916944
-
Radiation-Induced Autophagy in Human Pancreatic Cancer Cells is Critically Dependent on G2 Checkpoint Activation: A Mechanism of Radioresistance in Pancreatic Cancer.Int J Radiat Oncol Biol Phys. 2021 Sep 1;111(1):260-271. doi: 10.1016/j.ijrobp.2021.04.001. Epub 2021 Jun 7. Int J Radiat Oncol Biol Phys. 2021. PMID: 34112559
-
Lysosomes contribute to radioresistance in cancer.Cancer Lett. 2018 Dec 28;439:39-46. doi: 10.1016/j.canlet.2018.08.029. Epub 2018 Sep 11. Cancer Lett. 2018. PMID: 30217567 Review.
-
Autophagy and its function in radiosensitivity.Tumour Biol. 2015 Jun;36(6):4079-87. doi: 10.1007/s13277-015-3496-x. Epub 2015 May 7. Tumour Biol. 2015. PMID: 25946972 Review.
Cited by
-
mRNA Subtype of Cancer-Associated Fibroblasts Significantly Affects Key Characteristics of Head and Neck Cancer Cells.Cancers (Basel). 2022 May 3;14(9):2286. doi: 10.3390/cancers14092286. Cancers (Basel). 2022. PMID: 35565415 Free PMC article.
-
CT Radiomics and Whole Genome Sequencing in Patients with Pancreatic Ductal Adenocarcinoma: Predictive Radiogenomics Modeling.Cancers (Basel). 2022 Dec 16;14(24):6224. doi: 10.3390/cancers14246224. Cancers (Basel). 2022. PMID: 36551709 Free PMC article.
-
Pharmacogenetic Review: Germline Genetic Variants Possessing Increased Cancer Risk With Clinically Actionable Therapeutic Relationships.Front Genet. 2022 May 24;13:857120. doi: 10.3389/fgene.2022.857120. eCollection 2022. Front Genet. 2022. PMID: 35685436 Free PMC article. Review.
-
Impact of Smad4 and p53 mutations on the prognosis of patients with pancreatic ductal adenocarcinoma undergoing chemotherapy.Int J Clin Oncol. 2023 Nov;28(11):1511-1519. doi: 10.1007/s10147-023-02396-w. Epub 2023 Aug 18. Int J Clin Oncol. 2023. PMID: 37596505
-
Therapeutic Potential of Autophagy Modulation in Cholangiocarcinoma.Cells. 2020 Mar 4;9(3):614. doi: 10.3390/cells9030614. Cells. 2020. PMID: 32143356 Free PMC article. Review.
References
-
- Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. - PubMed
-
- Neoptolemos JP. Adjuvant treatment of pancreatic cancer. European journal of cancer. 2011;47 Suppl 3:S378–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

