Protocol for the mixed-methods process and context evaluation of the TB & Tobacco randomised controlled trial in Bangladesh and Pakistan: a hybrid effectiveness-implementation study
- PMID: 29602847
- PMCID: PMC5887198
- DOI: 10.1136/bmjopen-2017-019878
Protocol for the mixed-methods process and context evaluation of the TB & Tobacco randomised controlled trial in Bangladesh and Pakistan: a hybrid effectiveness-implementation study
Abstract
Introduction: Tuberculosis (TB) remains a significant public health problem in South Asia. Tobacco use increases the risks of TB infection and TB progression. The TB& Tobacco placebo-controlled randomised trial aims to (1) assess the effectiveness of the tobacco cessation medication cytisine versus placebo when combined with behavioural support and (2) implement tobacco cessation medication and behavioural support as part of general TB care in Bangladesh and Pakistan. This paper summarises the process and context evaluation protocol embedded in the effectiveness-implementation hybrid design.
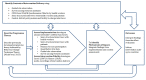
Methods and analysis: We are conducting a mixed-methods process and context evaluation informed by an intervention logic model that draws on the UK Medical Research Council's Process Evaluation Guidance. Our approach includes quantitative and qualitative data collection on context, recruitment, reach, dose delivered, dose received and fidelity. Quantitative data include patient characteristics, reach of recruitment among eligible patients, routine trial data on dose delivered and dose received, and a COM-B ('capability', 'opportunity', 'motivation' and 'behaviour') questionnaire filled in by participating health workers. Qualitative data include semistructured interviews with TB health workers and patients, and with policy-makers at district and central levels in each country. Interviews will be analysed using the framework approach. The behavioural intervention delivery is audio recorded and assessed using a predefined fidelity coding index based on behavioural change technique taxonomy.
Ethics and dissemination: The study complies with the guidelines of the Declaration of Helsinki. Ethics approval for the study and process evaluation was granted by the University of Leeds (qualitative components), University of York (trial data and fidelity assessment), Bangladesh Medical Research Council and Bangladesh Drug Administration (trial data and qualitative components) and Pakistan Medical Research Council (trial data and qualitative components). Results of this research will be disseminated through reports to stakeholders and peer-reviewed publications and conference presentations.
Trial registration number: ISRCTN43811467; Pre-results.
Keywords: clinical trials; epidemiology; organisation of health services; public health; qualitative research; tuberculosis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: EK, KZ and AP received payment for clinical studies and educational activities from Pfizer. They received no funding from a cytisine producing pharmaceutical company. KS received a research grant from Pfizer (2015–2017) to study the effect of varenicline (a smoking cessation medicine) on waterpipe smoking cessation.
Figures
References
-
- World Health Organization. Global Tuberculosis Report 2017: Leave no one behind - Unite to end TB. Geneva: World Health Organization, 2017.
-
- World Health Organization. Tuberculosis & Tobacco: a strong association. TB Tob. Factsheet. 2009. http://www.who.int/tobacco/resources/publications/factsheet_tb_tobacco_s... (accessed 30 May 2017).
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases