Methylene Blue Counteracts H2S-Induced Cardiac Ion Channel Dysfunction and ATP Reduction
- PMID: 29603116
- PMCID: PMC6126975
- DOI: 10.1007/s12012-018-9451-5
Methylene Blue Counteracts H2S-Induced Cardiac Ion Channel Dysfunction and ATP Reduction
Abstract
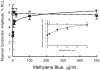
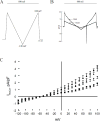
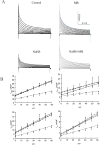
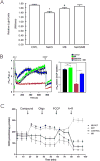
We have previously demonstrated that methylene blue (MB) counteracts the effects of hydrogen sulfide (H2S) cardiotoxicity by improving cardiomyocyte contractility and intracellular Ca2+ homeostasis disrupted by H2S poisoning. In vivo, MB restores cardiac contractility severely depressed by sulfide and protects against arrhythmias, ranging from bundle branch block to ventricular tachycardia or fibrillation. To dissect the cellular mechanisms by which MB reduces arrhythmogenesis and improves bioenergetics in myocytes intoxicated with H2S, we evaluated the effects of H2S on resting membrane potential (Em), action potential (AP), Na+/Ca2+ exchange current (INaCa), depolarization-activated K+ currents and ATP levels in adult mouse cardiac myocytes and determined whether MB could counteract the toxic effects of H2S on myocyte electrophysiology and ATP. Exposure to toxic concentrations of H2S (100 µM) significantly depolarized Em, reduced AP amplitude, prolonged AP duration at 90% repolarization (APD90), suppressed INaCa and depolarization-activated K+ currents, and reduced ATP levels in adult mouse cardiac myocytes. Treating cardiomyocytes with MB (20 µg/ml) 3 min after H2S exposure restored Em, APD90, INaCa, depolarization-activated K+ currents, and ATP levels toward normal. MB improved mitochondrial membrane potential (∆ψm) and oxygen consumption rate in myocytes in which Complex I was blocked by rotenone. We conclude that MB ameliorated H2S-induced cardiomyocyte toxicity at multiple levels: (1) reversing excitation-contraction coupling defects (Ca2+ homeostasis and L-type Ca2+ channels); (2) reducing risks of arrhythmias (Em, APD, INaCa and depolarization-activated K+ currents); and (3) improving cellular bioenergetics (ATP, ∆ψm).
Keywords: Arrhythmogenesis; Ion currents; Patch clamp; Sulfide toxicity.
Conflict of interest statement
No conflict of interest, financial and otherwise, is declared by the authors.
Figures

References
-
- Almeida AF, Guidotti TL. Differential sensitivity of lung and brain to sulfide exposure: a peripheral mechanism for apnea. Toxicol Sci. 1999;50:287–293. - PubMed
-
- Almgren T, Dyrssen D, Elgquist B, Johannsson O. Dissociation of hydrogen sulfide in seawater and comparison of pH scales. Marine Chemistry. 1976;4:289–297.
-
- Arnold IM, Dufresne RM, Alleyne BC, Stuart PJ. Health implication of occupational exposures to hydrogen sulfide. J Occup Med. 1985;27:373–376. - PubMed
-
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004;83:265–273. - PubMed
-
- Astier A, Baud FJ. Complexation of intracellular cyanide by hydroxocobalamin using a human cellular model. Human & experimental toxicology. 1996;15:19–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

