Impacts of human papillomavirus vaccination for different populations: A modeling study
- PMID: 29603224
- PMCID: PMC6099330
- DOI: 10.1002/ijc.31409
Impacts of human papillomavirus vaccination for different populations: A modeling study
Abstract
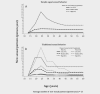
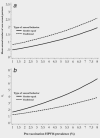
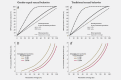
International variations in the prevalence of HPV infection derive from differences in sexual behaviors, which are also a key factor of the basic reproductive number (R0 ) of HPV infection in different populations. R0 affects the strength of herd protection and hence the impact of a vaccination program. Similar vaccination programs may therefore generate different levels of impact depending upon the population's pre-vaccination HPV prevalence. We used IARC's transmission model to estimate (i) the overall effectiveness of vaccination versus no vaccination in women aged 15-34 years measured as percent prevalence reduction (%PR) of HPV16 and (ii) the corresponding herd protection in populations with gender-equal or traditional sexual behavior and with different levels of sexual activity, corresponding to pre-vaccination HPV16 prevalence from 1 to 8% as observed worldwide. Between populations with different levels of gender-equal sexual activity, the highest difference in %PR under girls-only vaccination is observed at 40% coverage (91%PR vs. 48%PR for 1% and 8% pre-vaccination prevalence, respectively). HPV16 elimination is obtained with 55 and 97% coverage, respectively. To achieve desirable levels of HPV16 prevalence after vaccination, different levels of coverage are required in populations with different levels of pre-vaccination HPV16 prevalence, for example, in populations with gender-equal sexual behavior a decrease to 1/1000 HPV16 from pre-vaccination prevalence of 1 and 8% would require coverages of 37 and 96%, respectively. In traditional populations, corresponding coverages would need to be 28 and 93%, respectively. In conclusion, pre-vaccination HPV prevalence strongly influences herd immunity and helps predict the overall effectiveness of HPV vaccination.
Keywords: HPV prevalence; HPV vaccination; coverage threshold; herd effect.
© 2018 International Agency for Research on Cancer (IARC/WHO); licensed by UICC.
Figures

References
-
- IARC . Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100B:1–475. Accessed September 12, 2017 at http://monographs.iarc.fr/ENG/Monographs/vol100B/index.php. - PMC - PubMed
-
- Joura EA, Giuliano AR, Iversen OE, et al. A 9‐valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–23. - PubMed
-
- Lehtinen M, Dillner J. Clinical trials of human papillomavirus vaccines and beyond. Nat Rev Clin Oncol 2013;10:400–10. - PubMed
-
- Crosbie EJ, Einstein MH, Franceschi S, et al. Human papillomavirus and cervical cancer. Lancet 2013;382:889–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

