Involvement of serum-derived exosomes of elderly patients with bone loss in failure of bone remodeling via alteration of exosomal bone-related proteins
- PMID: 29603567
- PMCID: PMC5946082
- DOI: 10.1111/acel.12758
Involvement of serum-derived exosomes of elderly patients with bone loss in failure of bone remodeling via alteration of exosomal bone-related proteins
Abstract
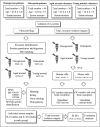
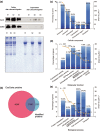
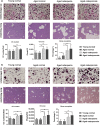
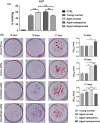
Exosomes are secreted into the blood by various types of cells. These extracellular vesicles are involved in the contribution of exosomal proteins to osteoblastic or osteoclastic regulatory networks during the failure of bone remodeling, which results in age-related bone loss. However, the molecular changes in serum-derived exosomes (SDEs) from aged patients with low bone density and their functions in bone remodeling remain to be fully elucidated. We present a quantitative proteomics analysis of exosomes purified from the serum of the elderly patients with osteoporosis/osteopenia and normal volunteers; these data are available via Proteome Xchange with the identifier PXD006463. Overall, 1,371 proteins were identified with an overlap of 1,160 Gene IDs among the ExoCarta proteins. Bioinformatics analysis and in vitro studies suggested that protein changes in SDEs of osteoporosis patients are not only involved in suppressing the integrin-mediated mechanosensation and activation of osteoblastic cells, but also trigger the differentiation and resorption of osteoclasts. In contrast, the main changes in SDEs of osteopenia patients facilitated both activation of osteoclasts and formation of new bone mass, which could result in a compensatory elevation in bone remodeling. While the SDEs from aged normal volunteers might play a protective role in bone health through facilitating adhesion of bone cells and suppressing aging-associated oxidative stress. This information will be helpful in elucidating the pathophysiological functions of SDEs and aid in the development of senile osteoporosis diagnostics and therapeutics.
Keywords: bone remodeling; exosome; osteoblasts; osteoclasts; osteoporosis.
© 2018 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures

References
-
- Barker, A. L. , McNeil, J. J. , Seeman, E. , Ward, S. A. , Sanders, K. M. , Khosla, S. , … Talevski, J. (2016). A randomised controlled trial of low‐dose aspirin for the prevention of fractures in healthy older people: Protocol for the ASPREE‐Fracture substudy. Injury Prevention: Journal of the International Society for Child and Adolescent Injury Prevention, 22, 297–301. 10.1136/injuryprev-2015-041655 - DOI - PMC - PubMed
-
- Chen, Y. , Xie, Y. , Xu, L. , Zhan, S. , Xiao, Y. , Gao, Y. , … Ge, W. (2017). Protein content and functional characteristics of serum‐purified exosomes from patients with colorectal cancer revealed by quantitative proteomics. International Journal of Cancer, 140, 900–913. 10.1002/ijc.30496 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

