Simultaneous targeting of EGFR, HER2, and HER4 by afatinib overcomes intrinsic and acquired cetuximab resistance in head and neck squamous cell carcinoma cell lines
- PMID: 29603584
- PMCID: PMC5983215
- DOI: 10.1002/1878-0261.12197
Simultaneous targeting of EGFR, HER2, and HER4 by afatinib overcomes intrinsic and acquired cetuximab resistance in head and neck squamous cell carcinoma cell lines
Abstract
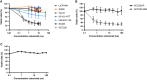
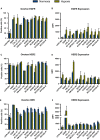
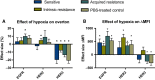
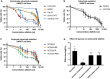
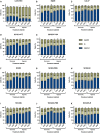
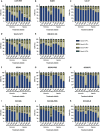
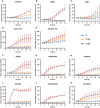
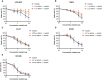
The epidermal growth factor receptor (EGFR, HER1) is a therapeutic target in head and neck squamous cell carcinoma (HNSCC). After initial promising results with EGFR-targeted therapies such as cetuximab, therapeutic resistance has become a major clinical problem, and new treatment options are therefore necessary. Moreover, the relationship between HER receptors, anti-EGFR therapies, and the human papillomavirus (HPV) status in HNSCC is not fully understood. In contrast to first-generation EGFR inhibitors, afatinib irreversibly inhibits multiple HER receptors simultaneously. Therefore, treatment with afatinib might result in a more pronounced therapeutic benefit, even in patients experiencing cetuximab resistance. In this study, the cytotoxic effect of afatinib as single agent and in combination with cisplatin was investigated in cetuximab-sensitive, intrinsically cetuximab-resistant, and acquired cetuximab-resistant HNSCC cell lines with different HPV status under normoxia and hypoxia. Furthermore, the influence of cetuximab resistance, HPV, and hypoxia on the expression of HER receptors was investigated. Our results demonstrated that afatinib was able to establish cytotoxicity in cetuximab-sensitive, intrinsically cetuximab-resistant, and acquired cetuximab-resistant HNSCC cell lines, independent of the HPV status. However, cross-resistance between cetuximab and afatinib might be possible. Treatment with afatinib caused a G0 /G1 cell cycle arrest as well as induction of apoptotic cell death. Additive to antagonistic interactions between afatinib and cisplatin could be observed. Neither cetuximab resistance nor HPV status significantly influenced the expression of HER receptors in HNSCC cell lines. In contrast, the expression of EGFR, HER2, and HER3 was significantly altered under hypoxia. Oxygen deficiency is a common characteristic of HNSCC tumors, and these hypoxic tumor regions often contain cells that are more resistant to treatment. However, we observed that afatinib maintained its cytotoxic effect under hypoxia. In conclusion, our preclinical data support the hypothesis that afatinib might be a promising therapeutic strategy to treat patients with HNSCC experiencing intrinsic or acquired cetuximab resistance.
Keywords: afatinib; cetuximab; epidermal growth factor receptor; head and neck squamous cell carcinoma; human papillomavirus; resistance.
© 2018 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Figures
Similar articles
-
The hypoxic tumor microenvironment and drug resistance against EGFR inhibitors: preclinical study in cetuximab-sensitive head and neck squamous cell carcinoma cell lines.BMC Res Notes. 2015 Jun 2;8:203. doi: 10.1186/s13104-015-1197-6. BMC Res Notes. 2015. PMID: 26032726 Free PMC article.
-
Dual Targeting of Epidermal Growth Factor Receptor and HER3 by MEHD7945A as Monotherapy or in Combination with Cisplatin Partially Overcomes Cetuximab Resistance in Head and Neck Squamous Cell Carcinoma Cell Lines.Cancer Biother Radiopharm. 2017 Sep;32(7):229-238. Cancer Biother Radiopharm. 2017. PMID: 28910149
-
Increased Expression of HER2, HER3, and HER2:HER3 Heterodimers in HPV-Positive HNSCC Using a Novel Proximity-Based Assay: Implications for Targeted Therapies.Clin Cancer Res. 2015 Oct 15;21(20):4597-606. doi: 10.1158/1078-0432.CCR-14-3338. Epub 2015 Jul 2. Clin Cancer Res. 2015. PMID: 26138066 Free PMC article.
-
Current challenges and clinical investigations of epidermal growth factor receptor (EGFR)- and ErbB family-targeted agents in the treatment of head and neck squamous cell carcinoma (HNSCC).Cancer Treat Rev. 2014 May;40(4):567-77. doi: 10.1016/j.ctrv.2013.10.002. Epub 2013 Oct 12. Cancer Treat Rev. 2014. PMID: 24216225 Review.
-
Irreversible multitargeted ErbB family inhibitors for therapy of lung and breast cancer.Curr Cancer Drug Targets. 2015;14(9):775-93. doi: 10.2174/1568009614666141111104643. Curr Cancer Drug Targets. 2015. PMID: 25435079 Review.
Cited by
-
Cetuximab-induced natural killer cell cytotoxicity in head and neck squamous cell carcinoma cell lines: investigation of the role of cetuximab sensitivity and HPV status.Br J Cancer. 2020 Sep;123(5):752-761. doi: 10.1038/s41416-020-0934-3. Epub 2020 Jun 16. Br J Cancer. 2020. PMID: 32541873 Free PMC article.
-
Emerging tyrosine kinase inhibitors for head and neck cancer.Expert Opin Emerg Drugs. 2022 Sep;27(3):333-344. doi: 10.1080/14728214.2022.2125954. Epub 2022 Sep 21. Expert Opin Emerg Drugs. 2022. PMID: 36131561 Free PMC article. Review.
-
Progress in the Research and Targeted Therapy of ErbB/HER Receptors in Urothelial Bladder Cancer.Front Mol Biosci. 2021 Dec 23;8:800945. doi: 10.3389/fmolb.2021.800945. eCollection 2021. Front Mol Biosci. 2021. PMID: 35004854 Free PMC article. Review.
-
The Role of Akt in Acquired Cetuximab Resistant Head and Neck Squamous Cell Carcinoma: An In Vitro Study on a Novel Combination Strategy.Front Oncol. 2021 Sep 10;11:697967. doi: 10.3389/fonc.2021.697967. eCollection 2021. Front Oncol. 2021. PMID: 34568028 Free PMC article.
-
The Right Partner in Crime: Unlocking the Potential of the Anti-EGFR Antibody Cetuximab via Combination With Natural Killer Cell Chartering Immunotherapeutic Strategies.Front Immunol. 2021 Sep 7;12:737311. doi: 10.3389/fimmu.2021.737311. eCollection 2021. Front Immunol. 2021. PMID: 34557197 Free PMC article. Review.
References
-
- Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK and Milas L (2002) Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Can Res 62, 7350–7356. - PubMed
-
- Boeckx C, Blockx L, de Beeck KO, Limame R, Camp GV, Peeters M, Vermorken JB, Specenier P, Wouters A, Baay M et al (2015a) Establishment and characterization of cetuximab resistant head and neck squamous cell carcinoma cell lines: focus on the contribution of the AP‐1 transcription factor. Am J Cancer Res 5, 1921–1938. - PMC - PubMed
-
- Boeckx C, Op de Beeck K, Wouters A, Deschoolmeester V, Limame R, Zwaenepoel K, Specenier P, Pauwels P, Vermorken JB, Peeters M et al (2014) Overcoming cetuximab resistance in HNSCC: the role of AURKB and DUSP proteins. Cancer Lett 354, 365–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

