Altered autonomic control of heart rate variability in the chronically hypoxic fetus
- PMID: 29604064
- PMCID: PMC6265555
- DOI: 10.1113/JP275659
Altered autonomic control of heart rate variability in the chronically hypoxic fetus
Abstract
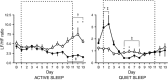
Key points: Fetal heart rate variability (FHRV) has long been recognised as a powerful predictor of fetal wellbeing, and a decrease in FHRV is associated with fetal compromise. However, the mechanisms by which FHRV is reduced in the chronically hypoxic fetus have yet to be established. The sympathetic and parasympathetic influences on heart rate mature at different rates throughout fetal life, and can be assessed by time domain and power spectral analysis of FHRV. In this study of chronically instrumented fetal sheep in late gestation, we analysed FHRV daily over a 16 day period towards term, and compared changes between fetuses of control and chronically hypoxic pregnancy. We show that FHRV in sheep is reduced by chronic hypoxia, predominantly due to dysregulation of the sympathetic control of the fetal heart rate. This presents a potential mechanism by which a reduction in indices of FHRV predicts fetuses at increased risk of neonatal morbidity and mortality in humans. Reduction in overall FHRV may therefore provide a biomarker that autonomic dysregulation of fetal heart rate control has taken place in a fetus where uteroplacental dysfunction is suspected.
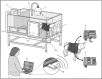
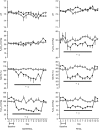
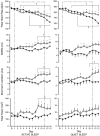
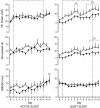
Abstract: Although fetal heart rate variability (FHRV) has long been recognised as a powerful predictor of fetal wellbeing, the mechanisms by which it is reduced in the chronically hypoxic fetus have yet to be established. In particular, the physiological mechanism underlying the reduction of short term variation (STV) in fetal compromise remains unclear. In this study, we present a longitudinal study of the development of autonomic control of FHRV, assessed by indirect indices, time domain and power spectral analysis, in normoxic and chronically hypoxic, chronically catheterised, singleton fetal sheep over the last third of gestation. We used isobaric chambers able to maintain pregnant sheep for prolonged periods in hypoxic conditions (stable fetal femoral arterial 10-12 mmHg), and a customised wireless data acquisition system to record beat-to-beat variation in the fetal heart rate. We determined in vivo longitudinal changes in overall FHRV and the sympathetic and parasympathetic contribution to FHRV in hypoxic (n = 6) and normoxic (n = 6) ovine fetuses with advancing gestational age. Normoxic fetuses show gestational age-related increases in overall indices of FHRV, and in the sympathetic nervous system contribution to FHRV (P < 0.001). Conversely, gestational age-related increases in overall FHRV were impaired by exposure to chronic hypoxia, and there was evidence of suppression of the sympathetic nervous system control of FHRV after 72 h of exposure to hypoxia (P < 0.001). This demonstrates that exposure to late gestation isolated chronic fetal hypoxia has the potential to alter the development of the autonomic nervous system control of FHRV in sheep. This presents a potential mechanism by which a reduction in indices of FHRV in human fetuses affected by uteroplacental dysfunction can predict fetuses at increased risk.
Keywords: Fetal Growth Restriction; Fetal heart rate; Fetal heart rate variability; Intrauterine hypoxia; Sympathetic nervous system.
© 2018 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures


Comment in
-
No sympathy for the hypoxic: the role of fetal oxygenation in autonomic dysfunction.J Physiol. 2018 Dec;596(23):5507-5508. doi: 10.1113/JP276227. Epub 2018 Jun 8. J Physiol. 2018. PMID: 29774552 Free PMC article. No abstract available.
References
-
- Assali NS, Brinkman CR 3rd, Woods JR Jr, Dandavino A & Nuwayhid B (1977). Development of neurohumoral control of fetal, neonatal, and adult cardiovascular functions. Am J Obstet Gynecol 129, 748–759. - PubMed
-
- Bennet L & Gunn AJ (2009). The fetal heart rate response to hypoxia: insights from animal models. Clin Perinatol 36, 655–672. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

