Maternal undernutrition in late gestation increases IGF2 signalling molecules and collagen deposition in the right ventricle of the fetal sheep heart
- PMID: 29604078
- PMCID: PMC6002234
- DOI: 10.1113/JP275806
Maternal undernutrition in late gestation increases IGF2 signalling molecules and collagen deposition in the right ventricle of the fetal sheep heart
Abstract
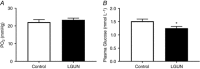
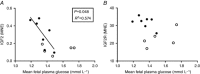
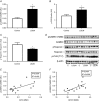
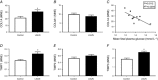
Key points: This study investigates the impact of decreased fetal plasma glucose concentrations on the developing heart in late gestation, by subjecting pregnant ewes to a 50% global nutrient restriction. Late gestation undernutrition (LGUN) decreased fetal plasma glucose concentrations whilst maintaining a normoxemic blood gas status. LGUN increased the mRNA expression of IGF2 and IGF2R. Fetal plasma glucose concentrations, but not fetal blood pressure, were significantly correlated with IGF2 expression and the activation of CAMKII in the fetal right ventricle. LGUN increased interstitial collagen deposition and altered the protein abundance of phospho-PLB and phospho-troponin I, regulators of cardiac contractility and relaxation. This study shows that a decrease in fetal plasma glucose concentrations may play a role in the development of detrimental changes in the right ventricle in early life, highlighting CAMKII as a potential target for the development of intervention strategies.
Abstract: Exposure of the fetus to a range of environmental stressors, including maternal undernutrition, is associated with an increased risk of death from cardiovascular disease in adult life. This study aimed to determine the effect of maternal nutrient restriction in late gestation on the molecular mechanisms that regulate cardiac growth and development of the fetal heart. Maternal undernutrition resulted in a decrease in fetal glucose concentrations across late gestation, whilst fetal arterial PO2 remained unchanged between the control and late gestation undernutrition (LGUN) groups. There was evidence of an up-regulation of IGF2/IGF2R signalling through the CAMKII pathway in the fetal right ventricle in the LGUN group, suggesting an increase in hypertrophic signalling. LGUN also resulted in an increased mRNA expression of COL1A, TIMP1 and TIMP3 in the right ventricle of the fetal heart. In addition, there was an inverse relationship between fetal glucose concentrations and COL1A expression. The presence of interstitial fibrosis in the heart of the LGUN group was confirmed through the quantification of picrosirius red-stained sections of the right ventricle. We have therefore shown that maternal undernutrition in late gestation may drive the onset of myocardial remodelling in the fetal right ventricle and thus has negative implications for right ventricle function and cardiac health in later life.
Keywords: IGF2; cardiac; fetus; fibrosis; heart; signalling; undernutrition.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures


Comment in
-
'Stiffening the sinews of the heart'.J Physiol. 2018 Jun;596(12):2279-2280. doi: 10.1113/JP276234. Epub 2018 May 10. J Physiol. 2018. PMID: 29676799 Free PMC article. No abstract available.
Similar articles
-
Impact of maternal late gestation undernutrition on surfactant maturation, pulmonary blood flow and oxygen delivery measured by magnetic resonance imaging in the sheep fetus.J Physiol. 2021 Oct;599(20):4705-4724. doi: 10.1113/JP281292. Epub 2021 Sep 22. J Physiol. 2021. PMID: 34487347
-
Late-gestation maternal undernutrition induces circulatory redistribution while preserving uteroplacental function independent of fetal glycaemic state.J Physiol. 2024 Dec;602(24):7065-7083. doi: 10.1113/JP287171. Epub 2024 Nov 16. J Physiol. 2024. PMID: 39549304
-
Fetal glucose availability: a key regulator of the metabolic, hormonal and contractility profiles of the fetal sheep heart.J Physiol. 2025 May 28. doi: 10.1113/JP288303. Online ahead of print. J Physiol. 2025. PMID: 40437764
-
Ontogeny and nutritional manipulation of the hepatic prolactin-growth hormone-insulin-like growth factor axis in the ovine fetus and in neonate and juvenile sheep.Proc Nutr Soc. 2004 Feb;63(1):127-35. doi: 10.1079/PNS2003324. Proc Nutr Soc. 2004. PMID: 15070443 Review.
-
Nutritional paradigms of ovine fetal growth restriction: implications for human pregnancy.Hum Fertil (Camb). 2005 Sep;8(3):179-87. doi: 10.1080/14647270500320121. Hum Fertil (Camb). 2005. PMID: 16234203 Review.
Cited by
-
Insulin-like Growth Factor-2 (IGF-2) in Fibrosis.Biomolecules. 2022 Oct 25;12(11):1557. doi: 10.3390/biom12111557. Biomolecules. 2022. PMID: 36358907 Free PMC article. Review.
-
Research Progress of Maternal Metabolism on Cardiac Development and Function in Offspring.Nutrients. 2023 Jul 30;15(15):3388. doi: 10.3390/nu15153388. Nutrients. 2023. PMID: 37571325 Free PMC article. Review.
-
Influence of the maternal high-intensity-interval-training on the cardiac Sirt6 and lipid profile of the adult male offspring in rats.PLoS One. 2020 Aug 3;15(8):e0237148. doi: 10.1371/journal.pone.0237148. eCollection 2020. PLoS One. 2020. PMID: 32745152 Free PMC article.
-
Feasibility of multimodal magnetic resonance imaging to assess maternal hyperoxygenation in sheep pregnancy.J Physiol. 2025 Mar;603(5):1029-1044. doi: 10.1113/JP287272. Epub 2025 Feb 12. J Physiol. 2025. PMID: 39937834 Free PMC article.
-
'Stiffening the sinews of the heart'.J Physiol. 2018 Jun;596(12):2279-2280. doi: 10.1113/JP276234. Epub 2018 May 10. J Physiol. 2018. PMID: 29676799 Free PMC article. No abstract available.
References
-
- Barbera A, Giraud GD, Reller MD, Maylie J, Morton MJ & Thornburg KL (2000). Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol 279, R1157–1164. - PubMed
-
- Barker DJ, Eriksson JG, Forsen T & Osmond C (2002). Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31, 1235–1239. - PubMed
-
- Barker DJ, Osmond C, Forsen TJ, Kajantie E & Eriksson JG (2005). Trajectories of growth among children who have coronary events as adults. N Engl J Med 353, 1802–1809. - PubMed
-
- Barker DJ, Winter PD, Osmond C, Margetts B & Simmonds SJ (1989). Weight in infancy and death from ischaemic heart disease. Lancet 2, 577–580. - PubMed
-
- Barker DJP ( 2000). In utero programming of cardiovascular disease. Theriogenology 53, 555–574. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

