Baseline characteristics and enrichment results from the SONAR trial
- PMID: 29604160
- PMCID: PMC6055730
- DOI: 10.1111/dom.13315
Baseline characteristics and enrichment results from the SONAR trial
Abstract
Aim: The SONAR trial uses an enrichment design based on the individual response to the selective endothelin receptor antagonist atrasentan on efficacy (the degree of the individual response in the urinary albumin-to-creatinine ratio [UACR]) and safety/tolerability (signs of sodium retention and acute increases in serum creatinine) to assess the effects of this agent on major renal outcomes. The patient population and enrichment results are described here.
Methods: Patients with type 2 diabetes with an estimated glomerular filtration rate (eGFR) within 25 to 75 mL/min/1.73 m2 and UACR between 300 and 5000 mg/g were enrolled. After a run-in period, eligible patients received 0.75 mg/d of atrasentan for 6 weeks. A total of 2648 responder patients in whom UACR decreased by ≥30% compared to baseline were enrolled, as were 1020 non-responders with a UACR decrease of <30%. Patients who experienced a weight gain of >3 kg and in whom brain natriuretic peptide exceeded ≥300 pg/mL, or who experienced an increase in serum creatinine >20% (0.5 mg/dL), were not randomized.
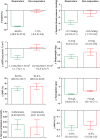
Results: Baseline characteristics were similar for atrasentan responders and non-responders. Upon entry to the study, median UACR was 802 mg/g in responders and 920 mg/g in non-responders. After 6 weeks of treatment with atrasentan, the UACR change in responders was -48.8% (95% CI, -49.8% to -47.9%) and in non-responders was -1.2% (95% CI, -6.4% to 3.9%). Changes in other renal risk markers were similar between responders and non-responders except for a marginally greater reduction in systolic blood pressure and eGFR in responders.
Conclusions: The enrichment period has successfully identified a population with a profound UACR reduction without clinical signs of sodium retention in whom a large atrasentan effect on clinically important renal outcomes is possible. The SONAR trial aims to establish whether atrasentan confers renal protection.
Keywords: atrasentan; diabetic kidney disease; endothelin receptor antagonist; precision medicine; randomized controlled clinical trial.
© 2018 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
H. J. L. H. is a consultant for AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Janssen, and Merck. He has a policy of all honoraria being paid to his employer.
D. d. Z. is a consultant for, and received honoraria (to employer) from AbbVie, Astellas, Bayer, Boehringer Ingelheim, Novo Nordisk, Fresenius, Janssen, and Mitsubishi Tanabe.
D. K. consults for AbbVie and has received grant support from the US National Institutes of Health (NIH). H.‐H. P. has equity in Merck and Novo Nordisk and has received consulting and lecture fees from AstraZeneca, Abbott, Novartis, and Reata. D. W. K. declares the following relationships: consultant for AbbVie, Relypsa, Corvia Medical, and Bayer; research grant funding from Novartis, Bayer, and St. Luke's Hospital of Kansas City; and stock ownership in Gilead Sciences. R. C. R. is a member of the Steering Committee of SONAR and has received honoraria from AbbVie, AstraZeneca, and Boehringer Ingelheim, and has lectured for Amgen, Takeda, AstraZeneca, and Roche. F. F. H. is a consultant for, and received honoraria from AbbVie and AstraZeneca. G. B. is a consultant for Bayer, Merck, KBP, Relypsa, Janssen, and AbbVie. G. B. is principal investigator of the FIDELIO trial (Bayer), and serves on steering committees for the CREDENCE (Janssen) and SONAR trials (AbbVie). H. M. is a consultant for AbbVie and Teijin, receives speaker honoraria from Astellas, Boehringer Ingelheim, MSD, and Tanabe Mitsubishi. J. M. declares the following relationships: consultant for AbbVie, Amgen, AstraZeneca, BMS, Cardiorentis, Dalcor, GlaxoSmithKline, Roche Pharmaceuticals, Merck, Novartis, Pfizer, Theracos, Oxford University/Bayer, and Kings College London/Vifor‐Fresenius Pharma. Honoraria are paid to his employer, Glasgow University. S. T. is a member of the steering committee of SONAR and has received consultant fees from AbbVie and Bayer, and has received honoraria from Servier and Valeant. V. P. serves on steering committees for trials funded by AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Janssen, and Pfizer; and serves on advisory boards and/or has spoken at scientific meetings for AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol‐Myers Squibb, Boehringer Ingelheim, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Roche, Sanofi, Servier, and Vitae. He has a policy of all honoraria being paid to his employer. J. J. B., J. D., D. L. A., T. T. Y. and M. W. are employees of AbbVie and may own stock or stock options.
Figures
References
-
- de Zeeuw D, Heerspink HJ. Unmet need in diabetic nephropathy: failed drugs or trials? Lancet Diabetes Endocrinol. 2016;4:638‐640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

