Requirement of zebrafish pcdh10a and pcdh10b in melanocyte precursor migration
- PMID: 29604249
- PMCID: PMC6163104
- DOI: 10.1016/j.ydbio.2018.03.022
Requirement of zebrafish pcdh10a and pcdh10b in melanocyte precursor migration
Abstract
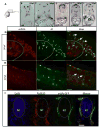
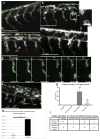
Melanocytes derive from neural crest cells, which are a highly migratory population of cells that play an important role in pigmentation of the skin and epidermal appendages. In most vertebrates, melanocyte precursor cells migrate solely along the dorsolateral pathway to populate the skin. However, zebrafish melanocyte precursors also migrate along the ventromedial pathway, in route to the yolk, where they interact with other neural crest derivative populations. Here, we demonstrate the requirement for zebrafish paralogs pcdh10a and pcdh10b in zebrafish melanocyte precursor migration. pcdh10a and pcdh10b are expressed in a subset of melanocyte precursor and somatic cells respectively, and knockdown and TALEN mediated gene disruption of pcdh10a results in aberrant migration of melanocyte precursors resulting in fully melanized melanocytes that differentiate precociously in the ventromedial pathway. Live cell imaging analysis demonstrates that loss of pchd10a results in a reduction of directed cell migration of melanocyte precursors, caused by both increased adhesion and a loss of cell-cell contact with other migratory neural crest cells. Also, we determined that the paralog pcdh10b is upregulated and can compensate for the genetic loss of pcdh10a. Disruption of pcdh10b alone by CRISPR mutagenesis results in somite defects, while the loss of both paralogs results in enhanced migratory melanocyte precursor phenotype and embryonic lethality. These results reveal a novel role for pcdh10a and pcdh10b in zebrafish melanocyte precursor migration and suggest that pcdh10 paralogs potentially interact for proper transient migration along the ventromedial pathway.
Keywords: Cell migration; Melanocytes; Neural crest; Protocadherin 10; Zebrafish.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures



References
-
- Baker KE, Parker R. Nonsense-mediated mRNA decay: terminating erroneous gene expression. Current Opinion in Cell Biology. 2004;16:293–299. - PubMed
-
- Bertrand KC, Mack SC, Northcott PA, Garzia L, Dubuc A, Pfister SM, Rutka JT, Weiss WA, Taylor MD. PCDH10 is a candidate tumour suppressor gene in medulloblastoma. Childs Nerv Syst. 2011;27:1243–1249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

