The impact of oxidative DNA damage and stress on telomere homeostasis
- PMID: 29604323
- PMCID: PMC6162185
- DOI: 10.1016/j.mad.2018.03.013
The impact of oxidative DNA damage and stress on telomere homeostasis
Abstract
Telomeres are dynamic nucleoprotein-DNA structures that cap and protect linear chromosome ends. Because telomeres shorten progressively with each replication, they impose a functional limit on the number of times a cell can divide. Critically short telomeres trigger cellular senescence in normal cells, or genomic instability in pre-malignant cells, which contribute to numerous degenerative and aging-related diseases including cancer. Therefore, a detailed understanding of the mechanisms of telomere loss and preservation is important for human health. Numerous studies have shown that oxidative stress is associated with accelerated telomere shortening and dysfunction. Oxidative stress caused by inflammation, intrinsic cell factors or environmental exposures, contributes to the pathogenesis of many degenerative diseases and cancer. Here we review the studies demonstrating associations between oxidative stress and accelerated telomere attrition in human tissue, mice and cell culture, and discuss possible mechanisms and cellular pathways that protect telomeres from oxidative damage.
Keywords: Base excision repair; Oxidative DNA damage; Oxidative stress; Telomeres.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest
Figures



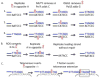
References
-
- Aeby E, Ahmed W, Redon S, Simanis V, Lingner J. Peroxiredoxin 1 Protects Telomeres from Oxidative Damage and Preserves Telomeric DNA for Extension by Telomerase. Cell Rep. 2016;17:3107–3114. - PubMed
-
- Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci. 2008;121:1046–1053. - PubMed
-
- Aikata H, Takaishi H, Kawakami Y, Takahashi S, Kitamoto M, Nakanishi T, Nakamura Y, Shimamoto F, Kajiyama G, Ide T. Telomere reduction in human liver tissues with age and chronic inflammation. Exp Cell Res. 2000;256:578–582. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

