A computational biology approach of a genome-wide screen connected miRNAs to obesity and type 2 diabetes
- PMID: 29605715
- PMCID: PMC6001404
- DOI: 10.1016/j.molmet.2018.03.005
A computational biology approach of a genome-wide screen connected miRNAs to obesity and type 2 diabetes
Abstract
Objective: Obesity and type 2 diabetes (T2D) arise from the interplay between genetic, epigenetic, and environmental factors. The aim of this study was to combine bioinformatics and functional studies to identify miRNAs that contribute to obesity and T2D.
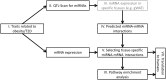
Methods: A computational framework (miR-QTL-Scan) was applied by combining QTL, miRNA prediction, and transcriptomics in order to enhance the power for the discovery of miRNAs as regulative elements. Expression of several miRNAs was analyzed in human adipose tissue and blood cells and miR-31 was manipulated in a human fat cell line.
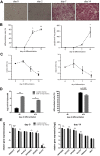
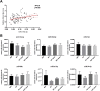
Results: In 17 partially overlapping QTL for obesity and T2D 170 miRNAs were identified. Four miRNAs (miR-15b, miR-30b, miR-31, miR-744) were recognized in gWAT (gonadal white adipose tissue) and six (miR-491, miR-455, miR-423-5p, miR-132-3p, miR-365-3p, miR-30b) in BAT (brown adipose tissue). To provide direct functional evidence for the achievement of the miR-QTL-Scan, miR-31 located in the obesity QTL Nob6 was experimentally analyzed. Its expression was higher in gWAT of obese and diabetic mice and humans than of lean controls. Accordingly, 10 potential target genes involved in insulin signaling and adipogenesis were suppressed. Manipulation of miR-31 in human SGBS adipocytes affected the expression of GLUT4, PPARγ, IRS1, and ACACA. In human peripheral blood mononuclear cells (PBMC) miR-15b levels were correlated to baseline blood glucose concentrations and might be an indicator for diabetes.
Conclusion: Thus, miR-QTL-Scan allowed the identification of novel miRNAs relevant for obesity and T2D.
Keywords: Adipogenesis; Computational biology; Insulin signalling; QTL; Type 2 diabetes; miR-31.
Copyright © 2018 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures






Similar articles
-
Picalm, a novel regulator of GLUT4-trafficking in adipose tissue.Mol Metab. 2024 Oct;88:102014. doi: 10.1016/j.molmet.2024.102014. Epub 2024 Aug 28. Mol Metab. 2024. PMID: 39182843 Free PMC article.
-
Liver microRNA transcriptome reveals miR-182 as link between type 2 diabetes and fatty liver disease in obesity.Elife. 2024 Jul 22;12:RP92075. doi: 10.7554/eLife.92075. Elife. 2024. PMID: 39037913 Free PMC article.
-
Dysregulation of Inflammation, Oxidative Stress, and Glucose Metabolism-Related Genes and miRNAs in Visceral Adipose Tissue of Women with Type 2 Diabetes Mellitus.Med Sci Monit. 2023 Jul 9;29:e939299. doi: 10.12659/MSM.939299. Med Sci Monit. 2023. PMID: 37422695 Free PMC article.
-
Regulatory microRNAs in Brown, Brite and White Adipose Tissue.Cells. 2020 Nov 16;9(11):2489. doi: 10.3390/cells9112489. Cells. 2020. PMID: 33207733 Free PMC article. Review.
-
Adipogenic miRNA and meta-signature miRNAs involved in human adipocyte differentiation and obesity.Oncotarget. 2016 Jun 28;7(26):40830-40845. doi: 10.18632/oncotarget.8518. Oncotarget. 2016. PMID: 27049726 Free PMC article. Review.
Cited by
-
Association of MiRNA Polymorphisms Involved in the PI3K/ATK/GSK3β Pathway with T2DM in a Chinese Population.Pharmgenomics Pers Med. 2025 Feb 14;18:71-84. doi: 10.2147/PGPM.S487873. eCollection 2025. Pharmgenomics Pers Med. 2025. PMID: 39974346 Free PMC article.
-
Polymorphisms in miRNA binding sites involved in metabolic diseases in mice and humans.Sci Rep. 2020 Apr 29;10(1):7202. doi: 10.1038/s41598-020-64326-4. Sci Rep. 2020. PMID: 32350386 Free PMC article.
-
Enriched Alternative Splicing in Islets of Diabetes-Susceptible Mice.Int J Mol Sci. 2021 Aug 10;22(16):8597. doi: 10.3390/ijms22168597. Int J Mol Sci. 2021. PMID: 34445304 Free PMC article.
-
Hyperglycemia Affects miRNAs Expression Pattern during Adipogenesis of Human Visceral Adipocytes-Is Memorization Involved?Nutrients. 2018 Nov 15;10(11):1774. doi: 10.3390/nu10111774. Nutrients. 2018. PMID: 30445791 Free PMC article.
-
miR-15b, a diagnostic biomarker and therapeutic target, inhibits oesophageal cancer progression by regulating the PI3K/AKT signalling pathway.Exp Ther Med. 2020 Dec;20(6):222. doi: 10.3892/etm.2020.9352. Epub 2020 Oct 15. Exp Ther Med. 2020. PMID: 33363587 Free PMC article.
References
-
- Golay A., Ybarra J. Link between obesity and type 2 diabetes. Best Practice & Research Clinical Endocrinology & Metabolism. 2005;19(4):649–663. - PubMed
-
- Davies J.L., Kawaguchi Y., Bennett S.T., Copeman J.B., Cordell H.J., Pritchard L.E. A genome-wide search for human type 1 diabetes susceptibility genes. Nature. 1994;371(6493):130–136. - PubMed
-
- McClellan J., King M.C. Genetic heterogeneity in human disease. Cell. 2010;141(2):210–217. - PubMed
-
- Peters L.L., Robledo R.F., Bult C.J., Churchill G.A., Paigen B.J., Svenson K.L. The mouse as a model for human biology: a resource guide for complex trait analysis. Nature Reviews Genetics. 2007;8(1):58–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

