Regulation of kynurenine metabolism by a ketogenic diet
- PMID: 29605816
- PMCID: PMC5983405
- DOI: 10.1194/jlr.M079251
Regulation of kynurenine metabolism by a ketogenic diet
Abstract
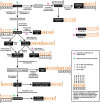
Ketogenic diets (KDs) are increasingly utilized as treatments for epilepsy, other neurological diseases, and cancer. Despite their long history in suppressing seizures, the distinct molecular mechanisms of action of KDs are still largely unknown. The goal of this study was to identify key metabolites and pathways altered in the hippocampus and plasma of rats fed a KD versus control diet (CD) either ad libitum or calorically restricted to 90% of the recommended intake. This was accomplished using a combination of targeted methods and untargeted MS-based metabolomics analyses. Various metabolites of and related to the tryptophan (TRP) degradation pathway, such as kynurenine (KYN), kynurenic acid as well as enzyme cofactors, showed significant changes between groups fed different diets and/or calorie amounts in plasma and/or the hippocampus. KYN was significantly downregulated in both matrices in animals of the CD-calorically restricted, KD-ad libitum, and KD-calorically restricted groups compared with the CD-ad libitum group. Our data suggest that the TRP degradation pathway is a key target of the KD.
Keywords: brain; caloric restriction; diet effects/lipid metabolism; epilepsy; kynurenic acid; kynurenine pathway; mass spectrometry; metabolomics; nutrition; tryptophan.
Copyright © 2018 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Dahlin M., Elfving A., Ungerstedt U., and Amark P.. 2005. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 64: 115–125. - PubMed
-
- Yamada K. A. 2008. Calorie restriction and glucose regulation. Epilepsia. 49 (Suppl. 8): 94–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

