Telerehabilitation for aphasia - protocol of a pragmatic, exploratory, pilot randomized controlled trial
- PMID: 29606148
- PMCID: PMC5880095
- DOI: 10.1186/s13063-018-2588-5
Telerehabilitation for aphasia - protocol of a pragmatic, exploratory, pilot randomized controlled trial
Abstract
Background: The Cochrane review on the effectiveness of speech and language therapy for aphasia following stroke suggests intensity of therapy is a key predictor for outcome. Current aphasia services cannot provide intervention at the intensity observed within trial contexts because of resource limitations. Telerehabilitation could widen access to speech-language pathologists (SLPs) in geographically remote contexts and reduce the time spent on travel by the therapist and patient. The current academic literature within this field is in its infancy, with few trials of speech and language therapy (SLT) delivered by videoconference. Our pilot randomized controlled trial (RCT) will explore feasibility aspects and effectiveness of telerehabilitation for aphasia in addition to standard SLT.
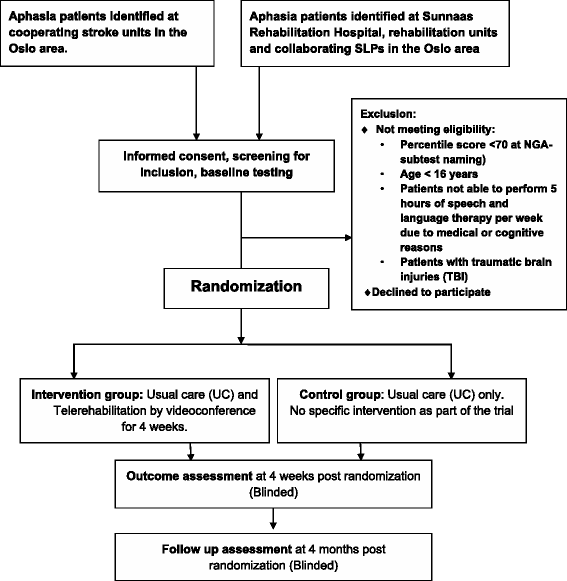
Method/design: Our study is a pragmatic, exploratory, pilot randomized controlled trial, where participants will be randomized to a telerehabilitation group or a control group. Both groups receive standard SLT (usual care) but the telerehabilitation group receives an additional 5 h of telerehabilitation per week over 4 weeks through videoconference. This additional telerehabilitation focuses on spoken language with an emphasis on word naming. We aim to include 40 patients in each group, with inclusion criteria being aphasia any time post stroke. Participants will be assessed blindly at pre-randomization (baseline), and 4 weeks and 4 months after randomization. The primary endpoint is naming ability 3 months after the completed intervention, measured by the Norwegian Basic Aphasia Assessment (NGA) naming subtest. Secondary endpoints include other subtests of the NGA, the VAST (Verb and Sentence Test) subtest sentence production, Communicative Effectiveness Index (CETI) and the Stroke and Aphasia Quality of Life scale (SAQOL-39). Experiences of patients and SLPs with telerehabilitation are assessed using questionnaires and semi-structured interviews. Statistical between group comparisons will be in line with an intention-to-treat analysis.
Discussion: This pilot RCT of intensive language training by videoconference will contribute new scientific evidence to the field of aphasia telerehabilitation. Here, we describe our trial which will explore the feasibility of telerehabilitation for aphasia as an intervention, our choice of primary and secondary outcome measures and proposed analyses. Our trial will provide information for the development and delivery of future definitive RCTs.
Trial registration: ClinicalTrials.gov, ID: NCT02768922 . Registered on 11 May 2016. Last updated on 17 November 2017.
Keywords: Aphasia; Intensive therapy; RCT design; Stroke; Telerehabilitation; Treatment efficacy; Videoconference.
Conflict of interest statement
Ethics approval and consent to participate
The study has received ethical approval by the Norwegian Regional Committee South East for Medical and Health Research Ethics. A Consent Form for participation in the trial has been developed and approved by the Ethics Committee. Informed consent will be obtained from all participants in the trial.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Ellekjaer H, Selmer R. Hjerneslag—like mange rammes, men prognosen er bedre. Tidsskr Nor Laegeforen. 2007;127:740–743. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical