GKAP Acts as a Genetic Modulator of NMDAR Signaling to Govern Invasive Tumor Growth
- PMID: 29606348
- PMCID: PMC5896248
- DOI: 10.1016/j.ccell.2018.02.011
GKAP Acts as a Genetic Modulator of NMDAR Signaling to Govern Invasive Tumor Growth
Abstract
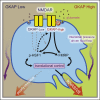
Genetic linkage analysis previously suggested that GKAP, a scaffold protein of the N-methyl-D-aspartate receptor (NMDAR), was a potential modifier of invasion in a mouse model of pancreatic neuroendocrine tumor (PanNET). Here, we establish that GKAP governs invasive growth and treatment response to NMDAR inhibitors of PanNET via its pivotal role in regulating NMDAR pathway activity. Combining genetic knockdown of GKAP and pharmacological inhibition of NMDAR, we implicate as downstream effectors FMRP and HSF1, which along with GKAP demonstrably support invasiveness of PanNET and pancreatic ductal adenocarcinoma cancer cells. Furthermore, we distilled genome-wide expression profiles orchestrated by the NMDAR-GKAP signaling axis, identifying transcriptome signatures in tumors with low/inhibited NMDAR activity that significantly associate with favorable patient prognosis in several cancer types.
Keywords: FMRP; GKAP/Dlgap1; GluN2b/NR2b/Grin2b; HSF1; MK801; NMDAR; RIP1Tag2; cancer modifier; glutamate receptor; memantine; pancreatic ductal adenocarcinoma (PDAC).
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Abbott L.F., Nelson S.B. Synaptic plasticity: taming the beast. Nat. Neurosci. 2000;3(Suppl):1178–1183. - PubMed
-
- Biton, A., Zinovyev, A., Barillot, E., and Radvanyi, F. (2013). MineICA: independent component analysis of transcriptomic data. https://www.bioconductor.org/packages/devel/bioc/vignettes/MineICA/inst/...
-
- Bostrom C.A., Majaess N.M., Morch K., White E., Eadie B.D., Christie B.R. Rescue of NMDAR-dependent synaptic plasticity in Fmr1 knock-out mice. Cereb. Cortex. 2015;25:271–279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

