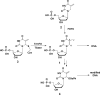
Chemo-enzymatic synthesis of the exocyclic olefin isomer of thymidine monophosphate
- PMID: 29606487
- PMCID: PMC5957510
- DOI: 10.1016/j.bmc.2018.03.032
Chemo-enzymatic synthesis of the exocyclic olefin isomer of thymidine monophosphate
Abstract
Exocyclic olefin variants of thymidylate (dTMP) recently have been proposed as reaction intermediates for the thymidyl biosynthesis enzymes found in many pathogenic organisms, yet synthetic reports on these materials are lacking. Here we report two strategies to prepare the exocyclic olefin isomer of dTMP, which is a putative reaction intermediate in pathogenic thymidylate biosynthesis and a novel nucleotide analog. Our most effective strategy involves preserving the existing glyosidic bond of thymidine and manipulating the base to generate the exocyclic methylene moiety. We also report a successful enzymatic deoxyribosylation of a non-aromatic nucleobase isomer of thymine, which provides an additional strategy to access nucleotide analogs with disrupted ring conjugation or with reduced heterocyclic bases. The strategies reported here are straightforward and extendable towards the synthesis of various pyrimidine nucleotide analogs, which could lead to compounds of value in studies of enzyme reaction mechanisms or serve as templates for rational drug design.
Keywords: Metabolism; Nucleotide analogue; Synthesis; Thymidylate.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The Authors declare no competing financial interest.
Figures
References
-
- Robertson KD. Nature Reviews Genetics. 2005;6:597. - PubMed
-
- Lakshminarasimhan R, Liang G. In: DNA Methyltransferases - Role and Function. Jeltsch A, Jurkowska RZ, editors. Springer International Publishing; Cham: 2016. p. 151.
-
- Rode AB, Kim BM, Park SH, Hong IS, Hong SH. Bioorg. Med. Chem. Lett. 2011;21:1151. - PubMed
-
- Chen XM, Wiemer DF. J. Org. Chem. 2003;68:6108. - PubMed
-
- Chen XM, Wiemer AJ, Hohl RJ, Wiemer DF. J. Org. Chem. 2002;67:9331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources