Construction and application of a liver cancer-targeting drug delivery system based on core-shell gold nanocages
- PMID: 29606870
- PMCID: PMC5868592
- DOI: 10.2147/IJN.S151043
Construction and application of a liver cancer-targeting drug delivery system based on core-shell gold nanocages
Abstract
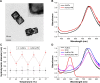
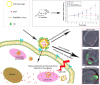
Background: In order to achieve drug targeting and controlled release, we have successfully developed a novel drug release system DOX/AuNCs-PM-HA with gold nanocages (AuNCs) as photothermal cores, thermally responsive copolymer P(NIPAM-co-Am) (PM) as the near-infrared (NIR) stimuli gatekeeper and hyaluronic acid as a targeting ligand as well as a capping agent.
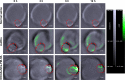
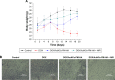
Methods: Cell uptake and cell viability were investigated. In vivo photoacoustic tomography imaging in H22 tumor bearing mice was analyzed for the tumor targeting effect of the nanocomplexes. Antitumor efficacy and the tissue distribution in vivo were investigated.
Results: In vitro results demonstrated that the DOX/AuNCs-PM-HA had significant anticancer activity against SMMC-7721 cells under NIR irradiation. Furthermore, in vivo photoacoustic tomography imaging of the nanocomplexes in H22 tumor bearing mice could indicate effective tumor targeting. Our studies on antitumor efficacy and the tissue distribution in vivo showed that many DOX/AuNCs-PM-HA nanocomplexes could efficiently accumulate at the tumor site so that they could inhibit the tumor growth effectively with limited side effects. The in vitro and in vivo results confirmed that the tumor-targeting and controlled-release drug system DOX/AuNCs-PM-HA with the combination of chemotherapy and photothermal therapy showed strong anti-tumor effect and would have great potential for future cancer therapy.
Conclusion: This tumor targeting DOX/AuNCs-PM-HA nanocomplex responded not only to the external stimuli of NIR, but also the internal stimuli of hyaluronidase, providing the potential for pinpointed and multi-stimuli responsive intracellular drug release.
Keywords: chemotherapy; drug delivery; hyaluronic acid; photoacoustic imaging; photothermal therapy; temperature-responsive polymers.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures






References
-
- Van EM, Murphy BP, Eufrásiodasilva T, et al. Nanomedicines for advanced cancer treatments: transitioning towards responsive systems. Int J Pharm. 2016;515(1–2):132–164. - PubMed
-
- Yang Y, Yang Y, Xie X, et al. Dual stimulus of hyperthermia and intracellular redox environment triggered release of siRNA for tumor-specific therapy. Int J Pharm. 2016;506(1–2):158–173. - PubMed
-
- Hu X, Wang Y, Zhang L, Man X, Wei D, Zhang J. Redox/pH dual stimuli-responsive degradable Salecan-g-SS-poly(IA-co-HEMA) hydrogel for release of doxorubicin. Carbohydr Polym. 2017;155:242–251. - PubMed
-
- Moreira AF, Dias DR, Correia IJ. Stimuli-responsive mesoporous silica nanoparticles for cancer therapy: a review. Microporous Mesoporous Mater. 2016;236:141–157.
-
- Farzin A, Fathi M, Emadi R. Multifunctional magnetic nanostructured hardystonite scaffold for hyperthermia, drug delivery and tissue engineering applications. Mater Sci Eng C. 2016;70:21–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

