Dysfunction of cortical synapse-specific mitochondria in developing rats exposed to lead and its amelioration by ascorbate supplementation
- PMID: 29606875
- PMCID: PMC5868605
- DOI: 10.2147/NDT.S148248
Dysfunction of cortical synapse-specific mitochondria in developing rats exposed to lead and its amelioration by ascorbate supplementation
Abstract
Background: Lead (Pb) is a widespread environmental neurotoxin and its exposure even in minute quantities can lead to compromised neuronal functions. A developing brain is particularly vulnerable to Pb mediated toxicity and early-life exposure leads to permanent alterations in brain development and neuronal signaling and plasticity, culminating into cognitive and behavioral dysfunctions and elevated risk of neuropsychiatric disorders later in life. Nevertheless, the underlying biochemical mechanisms have not been completely discerned.
Methods: Because of their ability to fulfill high energy needs and to act as calcium buffers in events of high intensity neuronal activity as well as their adaptive regulatory capability to match the requirements of the dynamicity of synaptic signaling, synapse-specific or synaptic mitochondria (SM) are critical for synaptic development, function and plasticity. Our aim for the present study hence was to characterize the effects of early-life Pb exposure on the functions of SM of prepubertal rats. For this purpose, employing a chronic model of Pb neurotoxicity, we exposed rat pups perinatally and postnatally to Pb and used a plethora of colorimetric and fluorometric assays for assessing redox and bioenergetic properties of SM. In addition, taking advantage of its ability as an antioxidant and as a metal chelator, we employed ascorbic acid (vitamin C) supplementation as an ameliorative therapeutic strategy against Pb-induced neurotoxicity and dysfunction of SM.
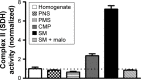
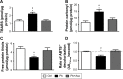
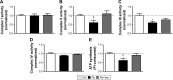
Results: Our results suggest that early-life exposure to Pb leads to elevated oxidative stress in cortical SM with consequent compromises in its energy metabolism activity. Ascorbate supplementation resulted in significant recovery of Pb-induced oxidative stress and functional compromise of SM.
Conclusion: Alterations in redox status and bioenergetic properties of SM could potentially contribute to the synaptic dysfunction observed in events of Pb neurotoxicity. Additionally, our study provides evidence for suitability of ascorbate as a significant ameliorative agent in tacking Pb neurotoxicity.
Keywords: heavy metal neurotoxicity; mitochondrial bioenergetics; mitochondrial membrane potential; neuropsychiatric; oxidative damage; synaptic.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Developmental lead (Pb)-induced deficits in hippocampal protein translation at the synapses are ameliorated by ascorbate supplementation.Neuropsychiatr Dis Treat. 2018 Nov 29;14:3289-3298. doi: 10.2147/NDT.S174083. eCollection 2018. Neuropsychiatr Dis Treat. 2018. PMID: 30568451 Free PMC article.
-
(Ascorb)ing Pb Neurotoxicity in the Developing Brain.Antioxidants (Basel). 2020 Dec 21;9(12):1311. doi: 10.3390/antiox9121311. Antioxidants (Basel). 2020. PMID: 33371438 Free PMC article. Review.
-
Developmental lead (Pb)-induced deficits in redox and bioenergetic status of cerebellar synapses are ameliorated by ascorbate supplementation.Toxicology. 2020 Jul;440:152492. doi: 10.1016/j.tox.2020.152492. Epub 2020 May 12. Toxicology. 2020. PMID: 32407874
-
Perinatal exposure to lead (Pb) induces ultrastructural and molecular alterations in synapses of rat offspring.Toxicology. 2016 Dec 12;373:13-29. doi: 10.1016/j.tox.2016.10.014. Epub 2016 Oct 29. Toxicology. 2016. PMID: 27974193
-
Glutamatergic components underlying lead-induced impairments in hippocampal synaptic plasticity.Neurotoxicology. 2000 Dec;21(6):1057-68. Neurotoxicology. 2000. PMID: 11233752 Review.
Cited by
-
Developmental lead (Pb)-induced deficits in hippocampal protein translation at the synapses are ameliorated by ascorbate supplementation.Neuropsychiatr Dis Treat. 2018 Nov 29;14:3289-3298. doi: 10.2147/NDT.S174083. eCollection 2018. Neuropsychiatr Dis Treat. 2018. PMID: 30568451 Free PMC article.
-
Maternal Lead Exposure Impairs Offspring Learning and Memory via Decreased GLUT4 Membrane Translocation.Front Cell Dev Biol. 2021 Feb 25;9:648261. doi: 10.3389/fcell.2021.648261. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33718391 Free PMC article.
-
Pre- and Neonatal Exposure to Lead (Pb) Induces Neuroinflammation in the Forebrain Cortex, Hippocampus and Cerebellum of Rat Pups.Int J Mol Sci. 2020 Feb 6;21(3):1083. doi: 10.3390/ijms21031083. Int J Mol Sci. 2020. PMID: 32041252 Free PMC article.
-
MOLECULAR MECHANISMS OF LEAD NEUROTOXICITY.Adv Neurotoxicol. 2021;5:159-213. doi: 10.1016/bs.ant.2020.11.002. Epub 2021 Feb 17. Adv Neurotoxicol. 2021. PMID: 34263090 Free PMC article.
-
(Ascorb)ing Pb Neurotoxicity in the Developing Brain.Antioxidants (Basel). 2020 Dec 21;9(12):1311. doi: 10.3390/antiox9121311. Antioxidants (Basel). 2020. PMID: 33371438 Free PMC article. Review.
References
-
- Meyer PA, Brown MJ, Falk H. Global approach to reducing lead exposure and poisoning. Mutat Res. 2008;659(1–2):166–175. - PubMed
-
- Chiodo LM, Jacobson SW, Jacobson JL. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol. 2004;26(3):359–371. - PubMed
-
- Allen KA. Is prenatal lead exposure a concern in infancy? What is the evidence? Adv Neonatal Care. 2015;15(6):416–420. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

