Synthesis, characterization, and stability study of desloratadine multicomponent crystal formation
- PMID: 29606963
- PMCID: PMC5842490
- DOI: 10.4103/1735-5362.223775
Synthesis, characterization, and stability study of desloratadine multicomponent crystal formation
Abstract
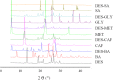
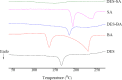
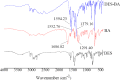
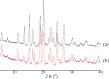
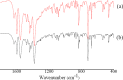
This study describes the formation of multicomponent crystal (MCC) of desloratadine (DES). The objective of this study was to discover the new pharmaceutical MCC of DES using several coformers. The MCC synthesis was performed between DES and 26 coformers using an equimolar ratio with a solvent evaporation technique. The selection of the appropriate solvent was carried out using 12 solvents. The preview of the MCC of DES was performed using polarized light microscopy (PLM). The formation of MCC was confirmed using powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC), fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM). The accelerated stability of MCC at 40 °C and relative humidity of 75% was investigated using PXRD and FTIR. Depending on the prior evaluation, DES and benzoic acid (BA) formed the MCC. PLM and SEM results showed that crystal habit of combination between DES and BA differed from the constituent components. Moreover, the diffractogram pattern of DES-BA was distinct from the constituent components. The DSC thermogram showed a new peak which was distinct from both constituent components. The FTIR study proved a new spectrum. All characterizations indicated that a new solid crystal was formed, ensuring the MCC formation. In addition, DES-BA MCC had both chemical and physical stabilities for a period of 4 months.
Keywords: Characterization; Desloratadine; Multicomponent crystal; Stability.
Figures


References
-
- Morissette SL, Almarsson Ö, Peterson ML, Remenar JF, Read MJ, Lemmo AV, et al. High-throughput crystallization: polymorphs, salts, cocrystals and solvates of pharmaceutical solids. Adv Drug Deliv Rev. 2004;56(3):275–300. - PubMed
-
- Basavoju S, Boström D, Velaga SP. Indomethacin-saccharin cocrystal: design, synthesis and preliminary pharmaceutical characterization. Pharm Res. 2008;25(3):530–541. - PubMed
-
- Singhal D, Curatolo W. Drug polymorphism and dosage form design: a practical perspective. Adv Drug Deliv Rev. 2004;56(3):335–347. - PubMed
-
- Serajuddin AT. Salt formation to improve drug solubility. Adv Drug Deliv Rev. 2007;59(7):603–616. - PubMed
-
- Duggirala NK, Perry ML, Almarsson Ö, Zaworotko MJ. Pharmaceutical cocrystals: along the path to improved medicines. Chem Commun (Camb) 2015;52(4):640–655. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

