Selection by parasitoid females among closely related hosts based on volatiles: Identifying relevant chemical cues
- PMID: 29607019
- PMCID: PMC5869356
- DOI: 10.1002/ece3.3877
Selection by parasitoid females among closely related hosts based on volatiles: Identifying relevant chemical cues
Abstract
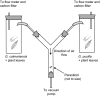
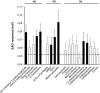
Parasitoid fitness is influenced by the ability to overcome host defense strategies and by the ability of parasitoid females to select high-quality host individuals. When females are unable to differentiate among hosts, their fitness will decrease with an increasing abundance of resistant hosts. To understand the effect of mixed host populations on female fitness, it is therefore necessary to investigate the ability of female parasitoids to select among hosts. Here, we used behavioral assays, headspace volatile collection, and electrophysiology to study the ability of Asecodes parviclava to use olfactory cues to select between a susceptible host (Galerucella calmariensis) and a resistant host (Galerucella pusilla) from a distance. Our studies show that parasitoid females have the capacity to distinguish the two hosts and that the selection behavior is acquired through experiences during earlier life stages. Further, we identified two volatiles (α-terpinolene and [E]-β-ocimene) which amounts differ between the two plant-herbivore systems and that caused behavioral and electrophysiological responses. The consequence of this selection behavior is that females have the capacity to avoid laying eggs in G. pusilla, where the egg mortality is higher due to much stronger immune responses toward A. parviclava than in larvae of G. calmariensis.
Keywords: Asecodes parviclava; electrophysiology; headspace volatile collection; host–parasitoid system; olfactometer.
Figures



References
-
- van Alphen, J. J. M. , & Drijver, R. A. B. (1982). Host selection by Asobara tabida Nees (Braconidae, Alysiinae) a larval parasitoid of fruit inhabiting Drosophila species I. Host stage selection with Drosophila melanogaster as host species. Netherlands Journal of Zoology, 32, 215–231.
-
- de Boer, J. G. , Hordijk, C. A. , Posthumus, M. A. , & Dicke, M. (2008). Prey and non‐prey arthropods sharing a host plant: Effects on induced volatile emission and predator attraction. Journal of Chemical Ecology, 34, 281–290. https://doi.org/10.1007/s10886-007-9405-z - DOI - PMC - PubMed
-
- Catherine, G. W. , Schulthess, F. , & Stephane, D. (2010). An association between host acceptance and virulence status of different populations of Cotesia sesamiae, a braconid larval parasitoid of lepidopteran cereal stemborers in Kenya. Biological Control, 54, 100–106. https://doi.org/10.1016/j.biocontrol.2010.04.010 - DOI
-
- Davis, J. M. , & Stamps, J. A. (2004). The effect of natal experience on habitat preferences. Trends in Ecology & Evolution, 19, 411–416. https://doi.org/10.1016/j.tree.2004.04.006 - DOI - PubMed
-
- De Moraes, C. M. , Lewis, W. J. , Pare, P. W. , Alborn, H. T. , & Tumlinson, J. H. (1998). Herbivore‐infested plants selectively attract parasitoids. Nature, 393, 570–573. https://doi.org/10.1038/31219 - DOI
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

