Intravital third harmonic generation microscopy of collective melanoma cell invasion: Principles of interface guidance and microvesicle dynamics
- PMID: 29607252
- PMCID: PMC5858865
- DOI: 10.4161/intv.21223
Intravital third harmonic generation microscopy of collective melanoma cell invasion: Principles of interface guidance and microvesicle dynamics
Abstract
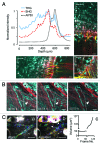
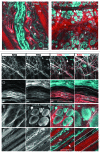
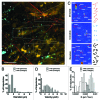
Cancer cell invasion is an adaptive process based on cell-intrinsic properties to migrate individually or collectively, and their adaptation to encountered tissue structure acting as barrier or providing guidance. Whereas molecular and physical mechanisms of cancer invasion are well-studied in 3D in vitro models, their topographic relevance, classification and validation toward interstitial tissue organization in vivo remain incomplete. Using combined intravital third and second harmonic generation (THG, SHG), and three-channel fluorescence microscopy in live tumors, we here map B16F10 melanoma invasion into the dermis with up to 600 µm penetration depth and reconstruct both invasion mode and tissue tracks to establish invasion routes and outcome. B16F10 cells preferentially develop adaptive invasion patterns along preformed tracks of complex, multi-interface topography, combining single-cell and collective migration modes, without immediate anatomic tissue remodeling or destruction. The data suggest that the dimensionality (1D, 2D, 3D) of tissue interfaces determines the microanatomy exploited by invading tumor cells, emphasizing non-destructive migration along microchannels coupled to contact guidance as key invasion mechanisms. THG imaging further detected the presence and interstitial dynamics of tumor-associated microparticles with submicron resolution, revealing tumor-imposed conditioning of the microenvironment. These topographic findings establish combined THG, SHG and fluorescence microscopy in intravital tumor biology and provide a template for rational in vitro model development and context-dependent molecular classification of invasion modes and routes.
Keywords: adipocyte; intravital multiphoton microscopy; melanoma; microparticles; myofiber; second harmonic generation; third harmonic generation; tumor invasion; tumor microenvironment.
Figures




References
-
- Sahai E. . Illuminating the metastatic process. Nat Rev Cancer 2007; 7:737 - 49; http://dx.doi.org/ 10.1038/nrc2229; PMID: 17891189 - DOI - PubMed
-
- Bravo-Cordero JJ, Hodgson L, Condeelis J. . Directed cell invasion and migration during metastasis. Curr Opin Cell Biol 2012; 24:277 - 83; http://dx.doi.org/ 10.1016/j.ceb.2011.12.004; PMID: 22209238 - DOI - PMC - PubMed
-
- Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, et al. . Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 2003; 160:267 - 77; http://dx.doi.org/ 10.1083/jcb.200209006; PMID: 12527751 - DOI - PMC - PubMed
-
- Friedl P, Gilmour D. . Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 2009; 10:445 - 57; http://dx.doi.org/ 10.1038/nrm2720; PMID: 19546857 - DOI - PubMed
-
- Friedl P, Wolf K. . Plasticity of cell migration: a multiscale tuning model. J Cell Biol 2010; 188:11 - 9; http://dx.doi.org/ 10.1083/jcb.200909003; PMID: 19951899 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
