Ischemic Preconditioning Promotes Autophagy and Alleviates Renal Ischemia/Reperfusion Injury
- PMID: 29607326
- PMCID: PMC5828321
- DOI: 10.1155/2018/8353987
Ischemic Preconditioning Promotes Autophagy and Alleviates Renal Ischemia/Reperfusion Injury
Abstract
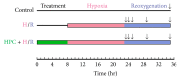
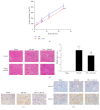
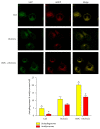
Autophagy is important for cellular survival during renal ischemia/reperfusion (I/R) injury. Ischemic preconditioning (IPC) has a strong renoprotective effect during renal I/R. Our study here aimed to explore the effect of IPC on autophagy during renal I/R injury. Rats were subjected to unilateral renal ischemia with or without prior IPC. Hypoxia/reoxygenation (H/R) injury was induced in HK-2 cells with or without prior hypoxic preconditioning (HPC). Autophagy and apoptosis were detected after reperfusion or reoxygenation for different time. The results showed that the levels of LC3II, Beclin-1, SQSTM1/p62, and cleaved caspase-3 were altered in a time-dependent manner during renal I/R. IPC further induced autophagy as indicated by increased levels of LC3II and Beclin-1, decreased level of SQSTM1/p62, and accumulation of autophagosomes compared to I/R groups at corresponding reperfusion time. In addition, IPC reduced the expression of cleaved caspase-3 and alleviated renal cell injury, as evaluated by the levels of serum creatinine (Scr), neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecule-1 (KIM-1) in renal tissues. In conclusion, autophagy and apoptosis are dynamically altered during renal I/R. IPC protects against renal I/R injury and upregulates autophagic flux, thus increasing the possibility for a novel therapy to alleviate I/R-induced acute kidney injury (AKI).
Figures


Similar articles
-
Ischemic preconditioning attenuates ischemia/reperfusion-induced kidney injury by activating autophagy via the SGK1 signaling pathway.Cell Death Dis. 2018 Mar 1;9(3):338. doi: 10.1038/s41419-018-0358-7. Cell Death Dis. 2018. PMID: 29497029 Free PMC article.
-
Ischemic preconditioning enhances autophagy but suppresses autophagic cell death in rat spinal neurons following ischemia-reperfusion.Brain Res. 2014 May 8;1562:76-86. doi: 10.1016/j.brainres.2014.03.019. Epub 2014 Mar 25. Brain Res. 2014. PMID: 24675029
-
Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys.Autophagy. 2019 Dec;15(12):2142-2162. doi: 10.1080/15548627.2019.1615822. Epub 2019 May 22. Autophagy. 2019. PMID: 31066324 Free PMC article.
-
[The role of pharmacological preconditioning in renal ischemic and reperfusion injury].Urologiia. 2017 Oct;(5):139-144. doi: 10.18565/urology.2017.5.139-144. Urologiia. 2017. PMID: 29135158 Review. Russian.
-
Is Renal Ischemic Preconditioning an Alternative to Ameliorate the Short- and Long-Term Consequences of Acute Kidney Injury?Int J Mol Sci. 2023 May 6;24(9):8345. doi: 10.3390/ijms24098345. Int J Mol Sci. 2023. PMID: 37176051 Free PMC article. Review.
Cited by
-
Total Glucosides of Paeony Inhibited Autophagy and Improved Acute Kidney Injury Induced by Ischemia-Reperfusion via the lncRNA TUG1/miR-29a/PTEN Axis.Drug Des Devel Ther. 2021 May 25;15:2229-2242. doi: 10.2147/DDDT.S286606. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34079224 Free PMC article.
-
Berberine protects steatotic donor undergoing liver transplantation via inhibiting endoplasmic reticulum stress-mediated reticulophagy.Exp Biol Med (Maywood). 2019 Dec;244(18):1695-1704. doi: 10.1177/1535370219878651. Epub 2019 Sep 25. Exp Biol Med (Maywood). 2019. PMID: 31554427 Free PMC article.
-
Aged kidneys are refractory to autophagy activation in a rat model of renal ischemia-reperfusion injury.Clin Interv Aging. 2019 Mar 1;14:525-534. doi: 10.2147/CIA.S197444. eCollection 2019. Clin Interv Aging. 2019. PMID: 30880933 Free PMC article.
-
Selenoprotein Gene mRNA Expression Evaluation During Renal Ischemia-Reperfusion Injury in Rats and Ebselen Intervention Effects.Biol Trace Elem Res. 2023 Apr;201(4):1792-1805. doi: 10.1007/s12011-022-03275-7. Epub 2022 May 12. Biol Trace Elem Res. 2023. PMID: 35553364
-
Ergosterol Protects Canine MDCK Cells from Gentamicin-Induced Damage by Modulating Autophagy and Apoptosis.Metabolites. 2025 Jun 5;15(6):373. doi: 10.3390/metabo15060373. Metabolites. 2025. PMID: 40559397 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

