Sirolimus enhances remission induction in patients with high risk acute myeloid leukemia and mTORC1 target inhibition
- PMID: 29607465
- PMCID: PMC6060002
- DOI: 10.1007/s10637-018-0585-x
Sirolimus enhances remission induction in patients with high risk acute myeloid leukemia and mTORC1 target inhibition
Abstract
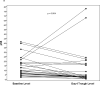
Background Mammalian Target of Rapamycin Complex 1 (mTORC1) inhibitors enhance chemotherapy response in acute myelogenous leukemia (AML) cells in vitro. However whether inhibiting mTORC1 enhances clinical response to AML chemotherapy remains controversial. We previously optimized measurement of mTORC1's kinase activity in AML blasts during clinical trials using serial phospho-specific flow cytometry of formaldehyde-fixed whole blood or marrow specimens. To validate mTORC1 as a therapeutic target in AML, we performed two clinical trials combining an mTORC1 inhibitor (sirolimus) and MEC (mitoxantrone, etoposide, cytarabine) in patients with relapsed, refractory, or untreated high-risk AML. Methods Flow cytometric measurements of ribosomal protein S6 phosphorylation (pS6) were performed before and during sirolimus treatment to determine whether mTORC1 inhibition enriched for chemotherapy response. Results In 51 evaluable subjects, the overall response rate (ORR) to the combination regimen was 47% (95% confidence interval 33-61%, 33% CR, 2% CRi, 12% PR) and similar toxicity to historic experience with MEC alone. 37 subjects had baseline pS6 measured pre-sirolimus, of whom 27 (73%) exhibited mTORC1 activity. ORR was not significantly different between subjects with and without baseline mTORC1 activity (52% vs 40%, respectively, p = 0.20). The ORR among subjects with baseline target activation and mTORC1 inhibition during therapy was 71% (12/17) compared to 20% (2/10) in subjects without target inhibition. Conclusions Fixed, whole blood pS6 by flow cytometry may be a predictive biomarker for clinical response to mTORC1 inhibitor-based regimens. These data provide clinical confirmation that mTORC1 activation mediates chemotherapy resistance in patients with AML.
Keywords: Acute myeloid leukemia; Biomarker; Phospho-flow cytometry; mTOR; mTORC1.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures





References
-
- Roboz GJ, Rosenblat T, Arellano M, Gobbi M, Altman JK, Montesinos P, O'Connell C, Solomon SR, Pigneux A, Vey N, Hills R, et al. International randomized phase iii study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2014 - PubMed
-
- Recher C, Beyne-Rauzy O, Demur C, Chicanne G, Dos Santos C, Mas VM, Benzaquen D, Laurent G, Huguet F, Payrastre B. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105(6):2527–2534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

