Inhaled anti-pseudomonal antibiotics for long-term therapy in cystic fibrosis
- PMID: 29607494
- PMCID: PMC8407188
- DOI: 10.1002/14651858.CD001021.pub3
Inhaled anti-pseudomonal antibiotics for long-term therapy in cystic fibrosis
Update in
-
Inhaled anti-pseudomonal antibiotics for long-term therapy in cystic fibrosis.Cochrane Database Syst Rev. 2022 Nov 14;11(11):CD001021. doi: 10.1002/14651858.CD001021.pub4. Cochrane Database Syst Rev. 2022. PMID: 36373968 Free PMC article.
Abstract
Background: Inhaled antibiotics are commonly used to treat persistent airway infection with Pseudomonas aeruginosa that contributes to lung damage in people with cystic fibrosis. Current guidelines recommend inhaled tobramycin for individuals with cystic fibrosis and persistent Pseudomonas aeruginosa infection who are aged six years or older. The aim is to reduce bacterial load in the lungs so as to reduce inflammation and deterioration of lung function. This is an update of a previously published review.
Objectives: To evaluate the effects long-term inhaled antibiotic therapy in people with cystic fibrosis on clinical outcomes (lung function, frequency of exacerbations and nutrition), quality of life and adverse events (including drug sensitivity reactions and survival).
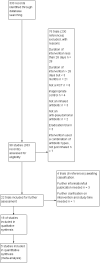
Search methods: We searched the Cochrane Cystic Fibrosis Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. We also searched ongoing trials registries.Date of last search: 13 February 2018.
Selection criteria: We selected trials if inhaled anti-pseudomonal antibiotic treatment was used for at least three months in people with cystic fibrosis, treatment allocation was randomised or quasi-randomised, and there was a control group (either placebo, no placebo or another inhaled antibiotic).
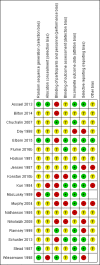
Data collection and analysis: Two authors independently selected trials, judged the risk of bias, extracted data from these trials and judged the quality of the evidence using the GRADE system.
Main results: The searches identified 333 citations to 98 trials; 18 trials (3042 participants aged between five and 56 years) met the inclusion criteria. Limited data were available for meta-analyses due to the variability of trial design and reporting of results. A total of 11 trials (1130 participants) compared an inhaled antibiotic to placebo or usual treatment for a duration between three and 33 months. Five trials (1255 participants) compared different antibiotics, two trials (585 participants) compared different regimens of tobramycin and one trial (90 participants) compared intermittent tobramycin with continuous tobramycin alternating with aztreonam. One of the trials (18 participants) compared to placebo and a different antibiotic and so fell into both groups. The most commonly studied antibiotic was tobramycin which was studied in 12 trials.We found limited evidence that inhaled antibiotics improved lung function (four of the 11 placebo-controlled trials, n = 814). Compared to placebo, inhaled antibiotics also reduced the frequency of exacerbations (three trials, n = 946), risk ratio 0.66 (95% confidence interval (CI) 0.47 to 0.93). There were insufficient data for us to be able to report an effect on nutritional outcomes or survival and there were insufficient data for us to ascertain the effect on quality of life. There was no significant effect on antibiotic resistance seen in the two trials that were included in meta-analyses. Tinnitus and voice alteration were the only adverse events significantly more common in the inhaled antibiotics group. The overall quality of evidence was deemed to be low for most outcomes due to risk of bias within the trials and imprecision due to low event rates.Of the eight trials that compared different inhaled antibiotics or different antibiotic regimens, there was only one trial in each comparison. Forced expiratory volume at one second (FEV1) % predicted was only found to be significantly improved with aztreonam lysine for inhalation compared to tobramycin (n = 273), mean difference -3.40% (95% CI -6.63 to -0.17). However, the method of defining the endpoint was different to the remaining trials and the participants were exposed to tobramycin for a long period making interpretation of the results problematic. No significant differences were found in the remaining comparisons with regard to lung function. Pulmonary exacerbations were measured in different ways, but one trial (n = 273) found that the number of people treated with antibiotics was lower in those receiving aztreonam than tobramycin, risk ratio 0.66 (95% CI 0.51 to 0.86). We found the quality of evidence for these comparisons to be directly related to the risk of bias within the individual trials and varied from low to high.
Authors' conclusions: Inhaled anti-pseudomonal antibiotic treatment probably improves lung function and reduces exacerbation rate, but pooled estimates of the level of benefit were very limited. The best evidence is for inhaled tobramycin. More evidence from trials measuring similar outcomes in the same way is needed to determine a better measure of benefit. Longer-term trials are needed to look at the effect of inhaled antibiotics on quality of life, survival and nutritional outcomes.
Conflict of interest statement
Nicola Rowbotham: received non‐financial supprot (travel and accomodation) for conference attendance from TEVA. Remaining authors: none known.
Figures
References
References to studies included in this review
Assael 2013 {published data only}
-
- Assael BM, Chiron R, Fischer R, Bresnick M, Lewis S, Montgomery AB, et al. Lung function improvement with aztreonam 75mg powder and solvent for nebuliser solution (AZLI) in single‐arm extension of a randomized trial of inhaled anti‐pseudomonal antibiotics in patients with cystic fibrosis (CF) and Pseudomonas aeruginosa infection. Journal of Cystic Fibrosis 2011;10 Suppl 1:S22. [Abstract no: 86; CFGD Register: PI257e]
-
- Assael BM, Chiron R, Fischer R, Bresnik M, Lewis S, Pressler T, et al. Lung function improvement with aztreonam for inhalation solution (AZLI) in the single‐arm extension of a randomized trials of inhaled anti‐pseudomonal antibiotics in patients with cystic fibrosis (CF) and Pseudomonas aeruginosa infection. Pediatric Pulmonology 2011;46 Suppl 34:312. [Abstract no: 278; CFGD Register: PI257c]
-
- Assael BM, Pressler T, Bilton D, Fayon M, Fischer R, Chiron R, et al. Inhaled aztreonam lysine vs. inhaled tobramycin in cystic fibrosis: a comparative efficacy trial. Journal of Cystic Fibrosis 2013;12(2):130‐40. [CFGD Register: PI257f] - PubMed
-
- Assael BM, Pressler T, Bilton D, Fayon M, Fischer R, Chiron R, et al. Inhaled aztreonam lysine vs. inhaled tobramycin in cystic fibrosis: a comparative efficacy trial. Journal of Cystic Fibrosis 2013;12(2):130‐40. Online figure 1. [CFGD Register: PI257k] - PubMed
-
- Assael BM, Pressler T, Bilton D, Fayon M, Fischer R, Chiron R, et al. Inhaled aztreonam lysine vs. inhaled tobramycin in cystic fibrosis: a comparative efficacy trial. Journal of Cystic Fibrosis 2013;12(2):130‐40. Online figure 2. [CFGD Register: PI257L] - PubMed
Bilton 2014 {published data only}
-
- Bilton D, Pressler T, Fajac I, Clancy JP, Sands D, Minic P, et al. Once‐daily liposomal amikacin for inhalation is non‐inferior to twice‐daily tobramycin inhalation solution in improving pulmonary function in cystic fibrosis patients with chronic infection due to Pseudomonas aeruginosa. Journal of Cystic Fibrosis 2014;13 Suppl 2:S15. [Abstract no: WS7.3; CFGD Register: PI271b]
-
- Bilton D, Pressler T, Fajac I, Clancy JP, Sands D, Minic PB, et al. Interim analysis of a long‐term, open‐label safety, tolerability, and efficacy study of liposomal amikacin for inhalation in cystic fibrosis patients with chronic infection due to pseudomonas aeruginosa. American Journal of Respiratory and Critical Care Medicine 2014;189(Meeting Abstracts). [Abstract no: A6571; CENTRAL: 1038420; CFGD Register: PI271c; CRS: 5500050000000490]
-
- Bilton D, Pressler T, Isabelle F, John Paul C, Sands D, Minic P, et al. Phase 3 efficacy and safety data from randomized, multicenter study of liposomal amikacin for inhalation (Arikace) compared with TOBI® in cystic fibrosis patients with chronic infection due to pseudomonas aeruginosa. Pediatric Pulmonology 2013;48 Suppl 36:290. [Abstract no: 235; CFGD Register: PI271a]
-
- Quittner AL, Barker DH, Constantine SE, Gupta R. Clinical benefit of liposomal amikacin for inhalation as assessed by the cystic fibrosis questionnaire‐revised. American Journal of Respiratory and Critical Care Medicine 2014;189(Meeting Abstracts). [Abstract no: A6685; CENTRAL: 1136702; CFGD Register: PI271d; CRS: 5500050000000489; EMBASE: 72047316]
Chuchalin 2007 {published data only}
-
- Chuchalin A, Csiszer E, Gyurkovics K, Bartnicka MT, Sands D, Kapranov N, et al. A formulation of aerosolized tobramycin (Bramitob) in the treatment of patients with cystic fibrosis and Pseudomonas aeruginosa infection: a double‐blind, placebo‐controlled, multicenter study. Paediatric Drugs 2007;9 Suppl 1:21‐31. [CFGD Register: PI201c] - PubMed
-
- Chuchalin A, Gyurkovics K, Mazurek H, Varoli G, Monici Preti P. Long‐term administration of aerosolised tobramycin in patients with cystic fibrosis. European Respiratory Journal 2005;26 Suppl 49:619s. [CFGD Register: PI201a]
-
- Chuchalin A, Gyurkovics K, Mazurek HVG, Monici Preti PA. Long‐term administration of nebulised tobramycin in patients with cystic fibrosis. Journal of Cystic Fibrosis 2006;5 Suppl:S42. [CFGD Register: PI201b]
Day 1988 {published data only}
-
- Day AJ, Williams J, McKeown C, Bruton A, Weller PH. Evaluation of inhaled colomycin in children with cystic fibrosis. 10th International Cystic Fibrosis Congress; 1988 March 5‐10; Sydney. 1988:106. [CFGD Register: PI85]
Elborn 2015 {published data only}
-
- Elborn JS, Flume PA, Cohen F, Loutit J, Devanter DR. Safety and efficacy of prolonged levofloxacin inhalation solution (APT‐1026) treatment for cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Journal of Cystic Fibrosis 2016;15(5):634‐40. [CENTRAL: 1262260; CFGD Register: PI262d; CRS: 5500135000001731; PUBMED: 26935334] - PubMed
-
- Elborn JS, Flume PA, Loutit J, Cohen F. Prolonged improvement in lung function and quality of life in cystic fibrosis: a 24‐week extension study of levofloxacin nebulization solution (APT‐1026) versus tobramycin nebulization solution in stable CF patients with chronic pseudomonas aeruginosa infection. Journal of Cystic Fibrosis 2014;13 Suppl 2:S16. [Abstract no: WS7.5; CENTRAL: 1000055; CFGD Register: PI262b; CRS: 5500131000000008]
-
- Elborn JS, Geller D, Conrad D, Aaron S, Smyth AR, Fischer R, et al. Phase 3 trial of inhaled levofloxacin (Aeruquinâ™, MP‐376, APT‐1026) vs. tobramycin inhalation solution (TIS) in intensively treated CF patients over 6 months. Journal of Cystic Fibrosis 2013;12 Suppl 1:S35. [Abstract no: WS17.6; CENTRAL: 867323; CFGD Register: PI262a; CRS: 5500100000011291]
-
- Elborn JS, Geller DE, Conrad D, Aaron SD, Smyth AR, Fischer R, et al. A phase 3, open‐label, randomized trial to evaluate the safety and efficacy of levofloxacin inhalation solution (APT‐1026) versus tobramycin inhalation solution in stable cystic fibrosis patients. Journal of Cystic Fibrosis 2015;14(4):507‐14. [CENTRAL: 1038490; CFGD Register: PI262c; CRS: 5500131000000327; DOI: 10.1016/j.jcf.2014.12.013; JID:: 101128966; PUBMED: 25592656] - DOI - PubMed
-
- Elborn JS, Geller DE, Conrad D, Aaron SD, Smyth AR, Fischer R, et al. A phase 3, open‐label, randomized trial to evaluate the safety and efficacy of levofloxacin inhalation solution (APT‐1026) versus tobramycin inhalation solution in stable cystic fibrosis patients. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14:507‐14. Online supplementary material. [CFGD Register: PI262e; CRS: 5500135000001735] - PubMed
Flume 2016b {published data only}
-
- Flume PA, Clancy JP, Retsch‐Bogart GZ, Tullis DE, Bresnik M, Derchak PA, et al. Continuous alternating inhaled antibiotics for chronic pseudomonal infection in cystic fibrosis. Journal of Cystic Fibrosis 2016;15(6):809‐15. [CENTRAL: 1262277; CFGD Register: PI288b; CRS: 5500135000001726; PUBMED: 27233377] - PubMed
-
- Flume PA, Clancy JP, Retsch‐Bogart GZ, Tullis E, Bresnik M, Derchak PA, et al. Aztreonam for inhalation solution (AZLI) and tobramycin inhalation solution (TIS) continuous alternating therapy (CAT) for cystic fibrosis (CF) patients with chronic pseudomonas aeruginosa (PA) infection: a randomized, double‐blind, placebo‐controlled trial. Pediatric Pulmonology 2015;50 Suppl 41:352. [Abstract no: 428; CENTRAL: 1092171; CFGD Register: PI288a; CRS: 5500135000001367]
Hodson 1981 {published data only}
-
- Hodson ME, Penketh ARL, Batten JC. Aerosol carbenicillin and gentamicin treatment of Pseudomonas aeruginosa infection in patients with cystic fibrosis. Lancet 1981;2(8256):1127‐9. [CFGD Register: PI24] - PubMed
Jensen 1987 {published data only}
-
- Jensen T, Pederson SS, Garne S, Heilmann C, Hoiby N, Koch C. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. Journal of Antimicrobial Chemotherapy 1987;19(6):831‐8. [CFGD Register: PI51] - PubMed
Konstan 2010b {published data only}
-
- Chiron R, Geller DE, Angyalosi G, Debonnett L, Yadao A, Bader G, et al. Tobramycin powder for inhalation is effective in advanced stage CF lung disease: the EAGER trial. Journal of Cystic Fibrosis 2014;13 Suppl 2:S57. [Abstract no: 42; CENTRAL: 996576; CFGD Register: PI239k; CRS: 5500129000000011]
-
- Geller DE, Flume PA, Brockhaus F, Zhang J, Angyalosi G, He E, et al. Treatment convenience and satisfaction of tobramycin inhalation powder (TIPTM) versus TOBI® in cystic fibrosis (CF) patients. Journal of Cystic Fibrosis 2010;9 Suppl 1:S22. [Abstract no: 83; CFGD Register: PI239b]
-
- Geller DE, Flume PA, Konstan M, Angyalosi G, Higgins M. Microbiological and clinical response to tobramycin inhalation powder (TIPTM) in cystic fibrosis patients with chronic Pseudomonas aeruginosa (Pa) infection. Journal of Cystic Fibrosis 2011;10 Suppl 1:S21. [Abstract no: 82; CFGD Register: PI239g]
-
- Geller DE, Nasr SZ, Piggott S, He E, Angyalosi G, Higgins M. Tobramycin inhalation powder in cystic fibrosis patients: response by age group. Respiratory Care 2014;59(3):388‐98. [CFGD Register: PI239l; CRS: 5500135000000283; PUBMED: 23983274] - PubMed
-
- Konstan M, Flume PA, Brockhaus F, Angyalosi G, He E, Geller D. Safety and efficacy of tobramycin inhalation powder (TIP) in treating CF patients infected with Pseudomonas aeruginosa (Pa). Journal of Cystic Fibrosis 2010;9 Suppl 1:S22. [Abstract no: 82; CFGD Register: PI239a]
Kun 1984 {published data only}
-
- Kun P, Landau LI, Phelan PD. Nebulized gentamicin in children and adolescents with cystic fibrosis. Australian Paediatric Journal 1984;20(1):43‐5. [CFGD Register: PI106] - PubMed
MacLusky 1989 {published data only}
-
- MacLusky IB, Gold R, Corey M, Levison H. Long‐term effects of inhaled tobramycin in patients with cystic fibrosis colonized with pseudomonas aeruginosa. Pediatric Pulmonology 1989;7(1):42‐8. [CFGD Register: PI62] - PubMed
Murphy 2004 {published data only}
-
- Anbar RD, Yu X, Colin AA. Reduction of pulmonary hospitalizations during a randomized, controlled, open‐label study of tobramycin solution for inhalation in young CF patients with mild lung disease. Pediatric Pulmonology 2003;Suppl 25:296. [CFGD Register: PI175b]
-
- Colin AA, Anbar RD, Yu X. Reduction in pulmonary hospitalizations during a randomized, controlled, open‐label study of tobramycin solution for inhalation in young CF patients with mild lung disease. Journal of Cystic Fibrosis 2003;2 Suppl 1:S22. [CFGD Register: PI175a]
-
- Murphy TD, Anbar RD, Lester LA, Nasr SZ, Nickerson B, Devanter DR, et al. Treatment with tobramycin solution for inhalation reduces hospitalizations in young CF subjects with mild lung disease. Pediatric Pulmonology 2004;38(4):314‐20. [CFGD Register: PI175c] - PubMed
Nathanson 1985 {published data only}
-
- Nathanson I, Cropp GJA, Li P, Neter P. Effectiveness of aerosolized gentamicin in cystic fibrosis (CF). Cystic Fibrosis Club Abstracts; 1985. 1985; Vol. 28:145. [CFGD Register: PI130]
Nikolaizik 2008 {published data only}
-
- Nikolaizik WH, Vietzke D, Ratjen F. Comparison of tobramycin 80 mg (IV‐preparation) and 300 mg solution inhaled twice daily for chronic P aeruginosa infection. Journal of Cystic Fibrosis 2005;4 Suppl:S53. [CFGD Register: PI191a]
Ramsey 1999 {published data only}
-
- Birnbaum HG, Greenberg P, Finkelstein S, Berndt E, Otto KL, Montgomery AB, et al. Economic analysis of hospitalization and home IV anti‐pseudomonal antibiotic use in CF patients on tobramycin solution for inhalation (TOBI). Pediatric Pulmonology 1998;Suppl 17:273. [CFGD Register: PI120f]
-
- Bowman CM. The long‐term use of inhaled tobramycin in patients with cystic fibrosis. Journal of Cystic Fibrosis 2002;1 Suppl 2:S194‐8. [CFGD Register: PI120cc] - PubMed
-
- Burns JL, Dalfsen JM, Shawar RM, Otto KL, Garber RL, Quan JM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. Journal of Infectious Diseases 1999;179(5):1190‐6. [CFGD Register: Pi120i] - PubMed
-
- Casey S, Ramsey B, Borowitz D. Nutritional benefits of chronic intermittent Pseudomonas aeruginosa suppression with tobramycin solution for inhalation in adolescents. 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm, Sweden. 2000:172. [CFGD Register: PI120ff]
-
- Enger C, Rothman K, Kylstra JW. Mortality rates during two years of treatment with intermittent inhaled tobramycin (TOBI) in CF. Pediatric Pulmonology 1999;Suppl 19:339‐40. [CFGD Register: PI120m]
Schuster 2013 {published data only}
-
- Goldman M, Schuster A, Halliburn C, Doering G, for the Freedom Study Group. A randomised, open label phase 3 study to evaluate the efficacy and safety of a dry powder formulation of inhaled colistimethate sodium (Colobreanthe®) verus tobramycin nebuliser solution (TNS) in cystic fibrosis subjects with chronic Pseudomonas aeruginosa lung infection. Journal of Cystic Fibrosis 2012;11 Suppl 1:S12. [Abstract no: WS5.5; CFGD Register: PI214b]
-
- Goldman MH, Pitt T. Lack of emergence of antimicrobial resistance of Pseudomonas aeruginosa after six months inhalation of dry powder colistimethate. Pediatric Pulmonology 2008;43 Suppl 31:331. [CFGD Register: PI214a]
-
- Goldman MH, Shuster A, Haliburn C, Doring G. A randomised, open label phase 3 study to evaluate the efficacy and safety of a dry powder formulation of colistimethate sodium (Colobreanthe®) verus tobramycin nebuliser solution (TNS) in cystic fibrosis subjects with chronic Pseudomonas aeruginosa lung infection. Pediatric Pulmonology 2012;47 Suppl 35:353. [Abstract no: 363; CFGD Register: PI214c]
-
- Goldman MH, Werner T, Schuster A. Does persistence with inhaled dry powder antibiotic treatment improve tolerability?. Pediatric Pulmonology 2013;48 Suppl 36:347. [Abstract no: 389; CENTRAL: 921669; CFGD Register: PI214f; CRS: 5500125000000396]
-
- Schuster A, Haliburn C, Doring G, Goldman MH, for the Freedom Study Group. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: a randomised study. Thorax 2013;68(4):344‐350. Online data supplement. [CFGD Register: PI214e; ; EudraCT: 2004‐003675‐36] - PMC - PubMed
Stead 1987 {published data only}
-
- Stead RJ, Hodson ME, Batten JC. Inhaled ceftazidime compared with gentamicin and carbenicillin in older patients with cystic fibrosis infected with Pseudomonas aeruginosa. British Journal of Diseases of the Chest 1987;81(3):272‐9. [CFGD Register: PI53b] - PubMed
-
- Stead RJ, Hodson ME, Batten JC. Nebulised ceftazidime compared with gentamicin and carbenicillin in adults with cystic fibrosis infected with Pseudomonas aeruginosa. 13th Annual Meeting of the European Working Group for Cystic Fibrosis; 1985 Nov 3‐8; Jerusalem. 1985; Vol. 2:51. [CFGD Register: PI53a]
Wiesemann 1998 {published data only}
-
- Ratjen F, Steinkamp G, Doring G, Bauernfeind A, Wiesemann HG, Hardt H. Prevention of chronic Pseudomonas aeruginosa infection by early inhalation therapy with tobramycin. Pediatric Pulmonology 1994;Suppl 10:250. [CFGD Register: PI101a]
-
- Wiesemann HG, Steinkamp G, Ratjen F, Bauernfeind A, Przyklenk B, Doring G, et al. Placebo‐controlled, double‐blind, randomized study of aerosolized tobramycin for early treatment of Pseudomonas aeruginosa colonization in cystic fibrosis. Pediatric Pulmonology 1998;25:88‐92. [CFGD Register: PI101b] - PubMed
References to studies excluded from this review
Al‐Aloul 2004 {published data only}
-
- Al‐Aloul M, Miller H, Browning P, Ledson MJ, Walshaw MJ. A randomised cross over trial of TOBI® vs IV tobramycin in acute pulmonary exacerbations in CF. Pediatric Pulmonology 2004;38 Suppl 27:249. [CFGD Register: PI186a]
-
- Al‐Aloul M, Miller H, Ledson MJ, Walshaw MJ. Tobramycin nebuliser solution (TOBI): a renal sparing alternative to intravenous (IV) tobramycin in acute pulmonary exacerbations in CF. Thorax 2004;59 Suppl II:ii79. [CENTRAL: 517107; CFGD Register: PI186d; CRS: 5500050000000402]
-
- Al‐Aloul M, Miller H, Ledson MJ, Walshaw MJ. Tobramycin nebuliser solution in the treatment of cystic fibrosis pulmonary exacerbations: effect on sputum pseudomonas aeruginosa density. Thorax 2005;2 Suppl 2:ii92. [CFGD Register: PI186b]
-
- Al‐Aloul M, Nazareth D, Walshaw M. Nebulized tobramycin in the treatment of adult cf pulmonary exacerbations. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2014;27(4):299‐305. [CFGD Register: PI186c] - PubMed
Alothman 2000 {published data only}
-
- Alothman GA, Alsaadi MM, Ho BL, Ho SL, Dupuis A, Corey M, et al. Evaluation of bronchial constriction in children with cystic fibrosis after inhaling two different preparations of tobramycin. Chest 2002;122(3):930‐4. [CFGD Register: PI157b] - PubMed
-
- Alothman GA, Coates Al, Corey M, Dupuis A, Ho SL, Ho BL, et al. In cystic fibrosis (CF) patients, does the inhalation of an intravenous tobramycin preparation result in more bronchospasm than a preservative free tobramycin preparation?. Pediatric Pulmonology 2000;Suppl 20:298‐9. [CFGD Register: PI157a]
Alothman 2005 {published data only}
-
- Alothman GA, Ho B, Alsaadi MM, Ho SL, O'Drowsky L, Louca E, et al. Bronchial constriction and inhaled colistin in cystic fibrosis. Chest 2005;127(2):522‐9. - PubMed
App 2000 {published data only}
-
- App EM, Huls G, Bittner‐Dersch P, Stolz S, Lindemann H, Matthys H. Impaired lung function influences the serum concentration of inhaled drugs in cystic fibrosis. Pediatric Pulmonology 2000;Suppl 20:279‐80. [CFGD Register: PI156b]
-
- Huls G, App EM, Bittner‐Dersch, Stolz S, Lindemann H. Impaired lung function influences the serum concentration of inhaled drugs in cystic fibrosis. 13th International Cystic Fibrosis Congress; 2000 Jun 4‐8; Stockholm. 2000:177. [CFGD Register: PI156a]
Bruinenberg 2008 {published data only}
-
- Bruinenberg P, Otulana B, Blanchard J, Morishige R, Cipolla D, Wilson J, et al. The effect of once‐a day inhaled liposomal ciprofloxacin hydrochloride on sputum bacterial density in cystic fibrosis patients with chronic pulmonary P aeruginosa colonization. Pediatric Pulmonology 2008;43 Suppl 31:344. [CFGD Register: PI216]
Carswell 1987 {published data only}
-
- Carswell F, Ward C, Cook DA, Speller DCE. A controlled trial of nebulized aminoglycoside and oral flucloxacillin versus placebo in the out‐patient management of children with cystic fibrosis. British Journal of Diseases of the Chest 1987;81(4):356‐60. [CFGD Register: PI54] - PubMed
Chua 1990 {published data only}
-
- Chua H, Collis G, Souef P. Bronchial response of children with cystic fibrosis to nebulised antibiotics. Australian and New Zealand Journal of Medicine 1990;20:537. [CFGD Register: PI66b]
-
- Chua HL, Collis GG, Souef PN. Bronchial response to nebulised antibiotics in children with cystic fibrosis. European Respiratory Journal 1990;3(10):1114‐6. [CFGD Register: PI66a] - PubMed
Coates 2011 {published data only}
-
- Coates AL, Denk O, Leung K, Ribeiro N, Chan J, Green M, et al. Higher tobramycin concentration and vibrating mesh technology can shorten antibiotic treatment time in cystic fibrosis. Pediatric Pulmonology 2011;46(4):401‐8. [CFGD Register: PI241b] - PubMed
-
- Denk O, Caotes AL, Keller M, Leung K, Green M, Chan J, et al. Lung delivery of a new tobramycin nebuliser solution (150mg/1.5ml) by an investigational eFlow® nebuliser is equivalent to TOBI® but in a fraction of time. Journal of Cystic Fibrosis 2009;8 Suppl 2:S66. [Abstract no: 264; CFGD Register: PI241c; ]
-
- Keller M, Coates AL, Griese M, Denk O, Schierholz J, Knoch M. In‐vivo data support equivalent therapeutic efficacy of a new tobramycin inhalation solution (150mg/1.5ml) administered by the eFlow® electronic nebuliser compared to TOBI® in the PARI LC PLUS®. Journal of Cystic Fibrosis 2010;9 Suppl 1:S22. [Abstract no: 84; CFGD Register: PI241a]
Cooper 1985 {published data only}
-
- Cooper DM, Harris M, Mitchell I. Comparison of intravenous and inhalation antibiotic therapy in acute pulmonary deterioration in cystic fibrosis. American Review of Respiratory Disease 1985;131. [Abstract no: A242; CFGD Register: PI129]
Davies 2004 {published data only}
-
- Conway SP, Davies JC, Etherington C, Goldman HH, Howard E, Jaffe A, et al. Piloting the use of the cystic fibrosis questionnaire (CFQ) in CF patients changing to dry powder inhaled colistimethate. Journal of Cystic Fibrosis 2007;6 Suppl 1:S76. [Abstract no: 309; CFGD Register: PI189b]
-
- Davies JC, Hall P, Francis J, Scott S, Geddes DM, Conway S, et al. A dry powder formulation of colistimethate sodium is safe and well tolerated in adults and children with CF. Pediatric Pulmonology 2004;38 Suppl 27:283. [Abstract no: 278; CFGD Register: PI189a]
-
- Goldman MH, Howard E, Lard N. FEV1% predicted may not be a simple end point for CF studies. Journal of Cystic Fibrosis 2007;6 Suppl 1:S34. [Abstract no: 141; CFGD Register: PI189c]
Dodd 1997 {published data only}
-
- Dodd M, Maddison J, Abbott J, Webb AK. The effect of the tonicity of nebulised colistin on lung function in adults with cystic fibrosis. 18th European Cystic Fibrosis Conference; 1993 May 21‐26; Madrid. 1993:121. [CFGD Register: PI100a]
-
- Dodd ME, Maddison J, Abbot J, Webb AR. The effect of the tonicity of nebulised colistin on chest tightness and lung function in adults with cystic fibrosis. European Respiratory Journal 1993;6 Suppl 17:515s. [CFGD Register: PI100c]
Dodd 1998 {published data only}
-
- Dodd ME, Haworth CS, Moorcroft AJ, Miles J, Webb AK. Is medicine evidence‐based when there is discrepancy between patient reported and objective measures of compliance in clinical trials?. Pediatric Pulmonology 1998;26 Suppl 17:389‐90. [CFGD Register: PI237]
Dorkin 2015 {published data only}
-
- Cystic Fibrosis Foundation. Inhaled ciprofloxacin. www.cff.org/clinicaltrials (accessed 17 Feb 2010). [CFGD Register: PI261c]
-
- Dorkin H, Criollo M, Reimnitz P, Alder J, Hampel B. Randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the safety and efficacy of inhaled ciprofloxacin compared with placebo in patients with cystic fibrosis‐ a phase IIB study of ciprofloxacin dry powder for inhalation (DPI). Pediatric Pulmonology 2011;46 Suppl 34:296. [Abstract no: 235; CFGD Register: PI261; CFGD Register: PI261a; ]
-
- NCT00645788. Study to evaluate the safety and efficacy of ciprofloxacin (inhaled) in patients with cystic fibrosis [Randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the safety and efficacy of inhaled ciprofloxacin compared to placebo in subjects with cystic fibrosis]. https://clinicaltrials.gov/ct2/show/NCT00645788 28 March 2008. [CFGD Register: PI261b; ClinicalTrials.gov identifier: NCT00645788]
Dupont 2008 {published data only}
-
- Clancy JP, Minic P, Dupont L, Goss CH, Quittner AL, Lymp JF, et al. Full analysis of data from two phase II blinded & placebo‐controlled studies of nebulized liposomal amikacin for inhalation (ArikaceTM) in the treatment of CF patients with Pseudomonas aeruginosa lung infection. Pediatric Pulmonology 2010;45 Suppl 33:299. [Abstract no: 227; CFGD Register: PI207d // PI255; ]
-
- Dupont L, Minic P, Fustic S, Mazurek H, Solyom E, Feketova A, et al. A randomised placebo‐controlled study of nebulized liposomal amikacin (Arikace™) in the treatment of cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. Journal of Cystic Fibrosis 2008;7 Suppl 2:S26. [Abstract no: 102; CFGD Register: PI207a]
-
- Dupont LJ, Clancy JP, Minic P, Goss CH, Fustic S, Mazurek Hetal. Evaluation of two phase II blinded and placebo‐controlled studies of nebulized liposomal amikacin (Arikace") in the treatment of cystic fibrosis patients with Pseudomonas aeruginosa lung infection. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts). [Abstract no: A1836; CENTRAL: 758757; CFGD Register: PI222e // PI207g ; CRS: 5500050000000487]
Eisenberg 1997 {published data only}
-
- Eisenberg J, Pepe M, Williams‐Warren J, Vasiliev M, Montgomery AB, Smith AL, et al. A comparison of peak sputum tobramycin concentration in patients with cystic fibrosis using jet and ultrasonic nebulizer systems. Chest 1997;111:955‐62. [CFGD Register: PI117] - PubMed
Flume 2016a {published data only}
-
- Flume P, Devanter DR, Cohen F, Fleming R, Elborn JS. Safety profile of levofloxacin inhalation solution from 3 controlled cystic fibrosis trials. Journal of Cystic Fibrosis 2015;14 Suppl 1:S87. [Abstract no: 117; CENTRAL: 1077213; CFGD Register: PI283c // PI240f // PI284c ; CRS: 5500135000001302]
-
- Flume PA, Devanter DR, Morgan EE, Dudley MN, Loutit JS, Bell SC, et al. A phase 3, multi‐center, multinational, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of levofloxacin inhalation solution (APT‐1026) in stable cystic fibrosis patients. Journal of Cystic Fibrosis 2016;15(4):495‐502. [CFGD Register: PI284d; CRS: 5500135000001727; PUBMED: 26852040] - PubMed
-
- Flume PA, Devanter DR, Morgan EE, Dudley MN, Loutit JS, Bell SC, et al. A phase 3, multi‐center, multinational, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of levofloxacin inhalation solution (APT‐1026) in stable cystic fibrosis patients. Journal of Cystic Fibrosis 2016;15(4):495‐502. Online supplement. [CFGD Register: PI284e; CRS: 5500135000001734] - PubMed
-
- NCT01180634. MP‐376 (Aeroquin™, levofloxacin for inhalation) in patients with cystic fibrosis [A phase 3, multi‐center, multinational, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of MP‐376 (Levofloxacin Inhalation Solution; Aeroquin™) in stable cystic fibrosis patients]. clinicaltrials.gov/show/NCT01180634 12 August 2010. [CENTRAL: 1012532; CFGD Register: PI284a; CRS: 5500131000000190]
-
- Devanter D, Flume PA, Fleming R, Elborn J. How often is pulmonary exacerbation defined by ≥4 Fuchs criteria associated with antibiotic treatment?. Pediatric Pulmonology 2014;49 Suppl 38:356. [Abstract no: 388; CENTRAL: 1012531; CFGD Register: PI283b // PI284b ; CRS: 5500131000000188]
Franz 1985 {published data only}
-
- Franz MN, Spohn WA, Ronald PM. Evaluation of tobramycin aerosol therapy in cystic fibrosis. Cystic Fibrosis Club Abstracts; 1985. 1985; Vol. 26:143.
Frederiksen 1997 {published data only}
-
- Frederiksen B, Hansen A, Koch C, Hoiby N. Delay of recurrence of Pseudomonas aeruginosa in patients with cystic fibrosis with inhaled colistin and oral ciprofloxacin; a comparison between 3 weeks and 3 months of treatment. Pediatric Pulmonology 1997;Supp 14:288. [CFGD Register: PI118a]
-
- Frederiksen B, Pressler T, Koch C, Hoiby N. Endpoints for evaluating early anti‐pseudomonal treatment: changes in pseudomonas prevalence and in pulmonary function. Pediatric Pulmonology 2003;Suppl 25:334. [CFGD Register: PI118b]
Galeva 2011 {published data only}
-
- Galeva I, Higgins M, Piggott S, Angyalosi G. A randomized, double‐blind, placebo‐controlled study of tobramycin inhalation powder in patients with cystic fibrosis: the edit trial. Pediatric Pulmonology 2011;46 Suppl 34:344. [Abstract no: 366; CFGD Register: PI260a; ]
-
- Galeva I, Konstan MW, Higgins M, Piggott S, Angyalosi G, Brockhaus F, et al. A challenging double‐blind, placebo‐controlled study of tobramycin inhalation powder in cystic fibrosis: results of the EDIT trial. Journal of Cystic Fibrosis 2012;11 Suppl 1:S12. [Abstract no: WS5.6; CFGD Register: PI260b ; ]
-
- Konstan M, Flume P, Wan R, Perrin M, Maykut R, Angyalosi G. Long‐term safety and efficacy of tobramycin inhalation powder in cystic fibrosis patients with P. aeruginosa: the EDIT trials and its two open‐label extension studies. Pediatric Pulmonology 2013;48 Suppl 36:344. [Abstract no: 381; CFGD Register: PI260d]
-
- Konstan MW, Flume PA, Galeva I, Wan R, Debonnett LM, Maykut RJ, et al. One‐year safety and efficacy of tobramycin powder for inhalation in patients with cystic fibrosis. Pediatric Pulmonology 2016;51(4):372‐8. [CENTRAL: 1260415; CFGD Register: PI260g; CRS: 5500135000001730; PUBMED: 26709158] - PubMed
Geborek 2003 {published data only}
-
- Geborek A, Hjelte L, Lindblad A, Mared L, Eriksson L, Johannesson M, et al. Cross‐over study of TOBI® vs. intravenous tobramycin in combination treatment of pulmonary exacerbations in cystic fibrosis patients. Journal of Cystic Fibrosis 2003;Suppl 1:S22. [CFGD Register: PI176]
Geller 2004 {published data only}
-
- Geller DE, Rodriguez CA, Howenstine M, Murphy T, Voter K, Nickerson B, et al. The effects of doubling concentration of tobramycin solution for inhalation on pharmacokinetics (PK), safety and delivery time in patients with cystic fibrosis (CF). American Journal of Respiratory and Critical Care Medicine 2004;169(7). [Abstract no: A391; CFGD Register: PI183a]
-
- Rosenfeld M, Geller DE, Rodriguez CA, Howenstine M, Konstan M, Ordonez C, et al. Serum pharmacokinetics of two preparations of tobramycin solution for inhalation in young cystic fibrosis patients. American Journal of Respiratory and Critical Care Medicine 2004;169(7). [Abstract no: A386; CFGD Register: PI183b]
Geller 2007 {published data only}
-
- Geller DE, Howenstine M, Conrad C, Smith J, Mulye S, Shrewsbury SB. A phase 1 study to assess the tolerability of a novel tobramycin powder for inhalation (TPI) formulation in cystic fibrosis subjects. Pediatric Pulmonology 2004;38 Suppl 27:250. [CFGD Register: PI187a]
-
- Geller DE, Konstan MW, Smith J, Noonberg SB, Conrad C. Novel tobramycin inhalation powder in cystic fibrosis subjects: pharmacokinetics and safety. Pediatric Pulmonology 2007;42(4):307‐13. [CFGD Register: PI187c] - PubMed
-
- Rodriguez CA, Shrewsbury SB, Potter SN, Nardella P, Geller DE. Single dose pharmacokinetics of tobramycin after administration of a novel dry powder formulation (TPI) in subjects with cystic fibrosis (CF). Pediatric Pulmonology 2004;38 Suppl 27:250. [CFGD Register: PI187b]
Geller 2011a {published data only}
-
- Geller DE, Flume PA, Griffith DC, Morgan E, White D, Loutit J, et al. Pharmacokinetics (PK) of aerosol MP‐376 (Aeroquin; levofloxacin inhalation solution) in CF patients. Journal of Cystis Fibrosis 2010;9 Suppl 1:S23. [Abstract no: 87; CFGD Register: PI254a; ]
Geller 2011b {published data only}
-
- Conrad D, Flume P, Sindel L, Andrews S, Morgan L, Loutit J, et al. Phase 2b study of inhaled MP‐376 (Aeroquin, levofloxacin inhalation solution) in stable cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa (PA) lung infection. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts). [CENTRAL: 1031676; CFGD Register: PI240g; CRS: 5500050000000360; EMBASE: 70839804]
-
- Flume P, Geller DE, Sindel L, Staab D, Fischer R, Riethmuller J, et al. Effects of inhaled MP‐376 (Aeroquin, levofloxacin inhalation solution) on lung function in stable cystic fibrosis (CF) patients with chronic pseudomonas aeruginosa (PA) lung function. Journal of Cystic Fibrosis 2010;9 Suppl 1:S23. [Abstract no: 86; CFGD Register: PI240a]
-
- Flume P, Devanter DR, Cohen F, Fleming R, Elborn JS. Safety profile of levofloxacin inhalation solution from 3 controlled cystic fibrosis trials. Journal of Cystic Fibrosis 2015;14 Suppl 1:S87. [Abstract no: 117; CENTRAL: 1077213; CFGD Register: PI240f // PI283c // PI284c ; CRS: 5500135000001302]
-
- Flume PA, Geller DE, Loutit JS, Dudley MN, Conrad D, Mpex 204 Study Group. Effects of inhaled MP‐376 (AeroquinTM, levofloxacin inhalation solution) on cystic fibrosis patients with both Staphylococcus aureus (SA) and Pseudomonas aeruginosa (PA) lung infection. Journal of Cystic Fibrosis 2011;10 Suppl 1:S22. [Abstract no: 87; CFGD Register: PI240b]
-
- Geller D, Flume PA, Sindel L, Staab D, Fischer R, Loutit J, et al. Effects of inhaled MP‐376 (Aeroquin, levofloxacin inhalation solution) on the need for other anti‐pseudomonal antimicrobials in stable CF patients with chronic Pseudomonas aeruginosa lung infection. Pediatric Pulmonology 2010;45 Suppl 33:301. [Abstract no: 232; CFGD Register: PI240c]
Gibson 2003 {published data only}
-
- Gibson RL, Emerson J, McNamara S. A randomized controlled trial of inhaled tobramycin in young children with cystic fibrosis: eradication of Pseudomonas from the lower airway. Pediatric Pulmonology 2002;Suppl 24:300. [CFGD Register: PI151c]
-
- Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2003;167(6):841‐9. [CFGD Register: PI151d] - PubMed
-
- Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2003;167(6):841. Online supplement. [CFGD Register: PI151e] - PubMed
-
- Rosenfeld M. Serum and lower respiratory tract tobramycin concentrations produced by inhaled tobramycin (TOBI) in young children with cystic fibrosis. Pediatric Pulmonology 1999;Suppl 19:106‐108. [CFGD Register: PI151b]
-
- Rosenfeld M, Borowitz D, Emerson J, Gibson R, McCoy K, McNamara S, et al. Serum pharmacokinetics and safety of inhaled tobramycin in very young CF patients. Pediatric Pulmonology 1999;Suppl 19:262. [CFGD Register: PI151s]
Gibson 2006 {published data only}
-
- Gibson RL, Retsch‐Bogart G, Ahrens R, Clancy J, Daines C, Milla C, et al. Safety and tolerability of aztreonam for inhalation (AI) in cystic fibrosis patients. Pediatric Pulmonology 2004;38 Suppl 27:253. [CFGD Register: PI188a]
-
- Gibson RL, Retsch‐Bogart GZ, Oermann C, Milla C, Pilewski J, Daines C, et al. Microbiology, safety, and pharmacokinetics of aztreonam lysinate for inhalation in patients with cystic fibrosis. Pediatric Pulmonology 2006;41(7):656‐65. [CFGD Register: PI188b] - PubMed
Goss 2009 {published data only}
-
- Clancy JP, Minic P, Dupont L, Goss CH, Quittner AL, Lymp JF, et al. Full analysis of data from two phase II blinded & placebo‐controlled studies of nebulized liposomal amikacin for inhalation (Arikace) in the treatment of CF patients with Pseudomonas aeruginosa lung infection. Pediatric Pulmonology 2010;45 Suppl 33:299. [Abstract no: 227; CFGD Register: PI222b // PI207d; ]
-
- Dupont LJ, Clancy JP, Minic P, Goss CH, Fustic S, Mazurek Hetal. Evaluation of two phase II blinded and placebo‐controlled studies of nebulized liposomal amikacin (Arikace") in the treatment of cystic fibrosis patients with Pseudomonas aeruginosa lung infection. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts). [Abstract no: A1836; CENTRAL: 758757; CFGD Register: PI222e // PI207g ; CRS: 5500050000000487]
-
- Goss CH, Clancy JP, Nick JA, Billings J, Rubenstein RC, Young KR, et al. A phase 2 blinded and placebo‐controlled study of nebulized liposomal amikacin (Arikace™) in the treatment of CF patients with pseudomonas aeruginosa lung infection. Pediatric Pulmonology 2009;44 Suppl 32:295. [Abstract no: 239; CFGD Register: PI222a]
Griffith 2008 {published data only}
-
- Geller DE, Flume P, Schwab R, Fornos P, Conrad DJ, Morgan E, et al. A phase 1 safety, tolerability and pharmacokinetic (PK) study of MP‐376 (levofloxacin solution for inhalation) in stable cystic fibrosis (CF) patients. Pediatric Pulmonology 2008;43 Suppl 31:315. [Abstract no: 321; CFGD Register: PI210b]
-
- Griffith DC, Hansen C, Pressler T, Balchen T, Jensen TJ, Geller DE, et al. Single‐dose pharmacokinetics of aerosol MP‐376 (levofloxacin solution for inhalation) in cystic fibrosis patients: PK‐PD implications. Journal of Cystic Fibrosis 2008;7 Suppl 2:S26. [CFGD Register: PI210a]
-
- Kearns GL, Rubino CM, Griffith DC, Geller DE, Forrest A, Bhavnani SM, et al. Levofloxacin pharmacokinetics (PK) after administration of MP‐376 (levofloxacin inhalation solution; Aeroquin) in children with cystic fibrosis. Journal of Cystic Fibrosis 2011;10 Suppl 1:S23. [Abstract no: 88; CFGD Register: PI210d]
-
- Stockmann C, Hillyard B, Ampofo K, Spigarelli MG, Sherwin CM. Levofloxacin inhalation solution for the treatment of chronic Pseudomonasaeruginosa infection among patients with cystic fibrosis. Expert Review of Respiratory Medicine 2015;9(1):13‐22. [CFGD Register: PI210c] - PubMed
Gulliver 2003 {published data only}
-
- Gulliver T, Wilson S, Williams G, Harris M, Cooper D. Nebulized tobramycin (intravenous solution) is tolerated without inducing cough and wheeze in cystic fibrosis patients. Thoracic Society of Australia & New Zealand Annual Scientific Meeting; 2003 April 4‐9; Adelaide, Australia. 2003. [Abstract no: P139; CFGD Register: PI184]
Hodson 2002 {published and unpublished data}
-
- Govan JR. Insights into cystic fibrosis microbiology from the European tobramycin trial in cystic fibrosis. Journal of Cystic Fibrosis 2002;1 Suppl 2:203‐8. [CFGD Register: PI153e] - PubMed
-
- Hodson ME, Gallagher CG. New clinical evidence from the European tobramycin trial in cystic fibrosis. Journal of Cystic Fibrosis 2002;1 Suppl 2:S199‐S202. [CFGD Register: PI153d] - PubMed
-
- Hodson ME, Gallagher CG, Govan JRW. A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. European Respiratory Journal 2002;20(3):658‐64. [CFGD Register: PI153c] - PubMed
-
- Hodson ME, Gallagher CG, Govan JRW, PL TNDS 101 Clinical Trial Group. Randomised UK / Eire clinical trial of the efficacy and safety of tobramycin 300 mg/5 ml nebuliser solution or nebulised colistin in CF patients. Pediatric Pulmonology 2000;Suppl 20:248‐9. [CFGD Register: PI145b]
-
- Hodson ME, Gallagher CG, Govan JRW, the PL‐TNDS‐101 Clinical Trial Group. Randomised UK/Eire clinical trial of the efficacy and safety of tobramycin 300 mg/5 mL nebuliser solution or nebulised colistin. 13th International Cystic Fibrosis Congress; 2000 Jun 4‐8; Stockholm. 2000:145. [CFGD Register: PI145a]
Jenkins 1985 {published data only}
-
- Jenkins SG, Kelly WC, Mason WG, Peele JD, Cruse MA, Coludro EO, et al. Aerosolized amikacin administration to cystic fibrosis patients chronically infected with Pseudomonas aeruginosa. Cystic Fibrosis Club Abstracts; 1985. 1985; Vol. 28:147. [CFGD Register: PI131]
Knowles 1988 {published data only}
-
- Knowles NE, Hallberg TK, Colombe JL. Efficacy of aerosolized antibiotics combined with intravenous treatment of cystic fibrosis pulmonary exacerbation. Pediatric Pulmonology 1998;Suppl 2(1):120. [CFGD Register: PI87]
Konstan 2010a {published data only}
-
- Konstan MW, Geller DE, Brockhaus F, Zhang J, Angyalosi G. Tobramycin inhalation powder is effective and safe in the treatment of chronic pulmonary Pseudomonas aeruginosa (Pa) infection in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2009;179. [Abstract no: A1186; CFGD Register: PI227a]
-
- Konstan MW, Geller DE, Minic P, Brockhaus F, Zhang J, Angyalosi G. Effective treatment of chronic Pseudomonas aeruginosa (Pa) infection with tobramycin inhalation powder in CF patients. Journal of Cystic Fibrosis 2009;8 Suppl 2:S27. [Abstract no: 105; CFGD Register: PI227b]
-
- McColley S, Rietschel E, Brockhaus F, Angyalosi G, Higgins M. Safety of inhaled tobramycin in patients with cystic fibrosis. Pediatric Pulmonology 2011;46 Suppl 34:344. [Abstract no: 365; CFGD Register: PI239h // PI227d ; ]
-
- NCT00125346. Tobramycin inhalation powder (TIP) in cystic fibrosis subjects (EVOLVE) [A randomized, double‐blind, placebo‐controlled, multicenter, phase 3 trial to assess the efficacy and safety of tobramycin inhalation powder (TIP) in cystic fibrosis (CF) subjects]. clinicaltrials.gov/ct2/show/NCT00125346 01 August 2005. [CFGD Register: PI227c]
Ledson 2002 {published data only}
-
- Ledson MJ, Gallagher MJ, Cowperthwaite C, Robinson M, Convery RP, Walshaw MJ. A randomised double blind placebo controlled crossover trial of nebulised taurolidine in adult CF patients colonised with B cepacia. 22nd European CF Conference; 1998 June 13‐19; Berlin, Germany. 1998:120. [CFGD Register: PI128a]
-
- Ledson MJ, Gallagher MJ, Robinson M, Cowperthwaite C, Willets T, Hart CA, Walshaw MJ. A randomized double‐blinded placebo‐controlled crossover trial of nebulized taurolidine in adult cystic fibrosis patients infected with Burkholderia cepacia. Journal of Aerosol Medicine 2002;15(1):51‐7. [CFGD Register: PI128b] - PubMed
Lenoir 2007 {published data only}
-
- Lenoir G, Antypkin YG, Miano A, Moretti P, Zanda M, Varoli G, et al. Efficacy, safety, and local pharmacokinetics of highly concentrated nebulized tobramycin in patients with cystic fibrosis colonized with Pseudomonas aeruginosa. Paediatric Drugs 2007;9 Suppl 1:11‐20. [CFGD Register: PI196c] - PubMed
-
- Lenoir G, Aryayev N, Varoli G, Monici Preti P. Aerosolized tobramycin in the treatment of patients with cystic fibrosis and pseudomonas aeruginosa infection. Journal of Cystic Fibrosis 2006;5 Suppl:S42. [CFGD Register: PI196b]
-
- Lenoir G, Aryayev N, Varoli G, Monici Preti P. Highly concentrated aerosolized tobramycin in the treatment of patients with cystic fibrosis and Pseudomonas aeruginosa infection. European Respiratory Journal 2005;26 Suppl 49:620s. [CFGD Register: PI196a]
Mainz 2014 {published data only}
-
- Mainz JG, Schadlich K, Schien C, Michl R, Schelhorn‐Neise P, Koitschev A, et al. Sinonasal inhalation of tobramycin vibrating aerosol in cystic fibrosis patients with upper airway Pseudomonas aeruginosa colonization: results of a randomized, double‐blind, placebo‐controlled pilot study. Drug Design, Development and Therapy 2014;8:209‐17. [CFGD Register: PI248b] - PMC - PubMed
-
- Mainz JG, Schien C, Schadlich K, Pfister W, Schelhorn‐Neise P, Koitschev A, et al. Sinonasal inhalation of tobramycin in cystic fibrosis patients with P. aeruginosa colonization of the upper airways ‐ results of a multicentric placebo‐controlled pilot study. Journal of Cystic Fibrosis 2011;10 Suppl 1:S21. [Abstract no: 83; CFGD Register: PI248a]
-
- Mainz JG, Schiller I, Ritschel C, Mentzel HJ, Riethmüller J, Koitschev A, et al. Sinonasal inhalation of dornase alfa in CF: A double‐blind placebo‐controlled cross‐over pilot trial. Auris, Nasus, Larynx 2011;38(2):220‐7. [CENTRAL: 781345; CFGD Register: PI248c; CRS: 5500050000000201; PUBMED: 21030168] - PubMed
Mazurek 2014 {published data only}
-
- Cicirello H, Mazurek H, Chiron R, Pelikan L, Geidel C, Bolbas K, et al. Efficacy and safety of two inhaled tobramycin solutions in patients with cystic fibrosis and chronic Pseudomonas aeruginosa infection: results from a head to head comparison. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A3121. [CENTRAL: 1031434; CFGD Register: PI249e; CRS: 5500050000000530; EMBASE: 70850327]
-
- Mazurek H, Chiron R, Kucerova T, Geidel C, Bolbas K, Chuchalin A, et al. Long‐term efficacy and safety of aerosolized tobramycin 300 mg/4 ml in cystic fibrosis. Pediatric Pulmonology 2014;49(11):1076‐89. [CENTRAL: 1015252; CFGD Register: PI249d; CRS: 5500131000000251; JID:: 8510590; PUBMED: 24464974] - PubMed
-
- Mazurek H, Chiron R, Pelikan L, Geidel C, Bolbas K, Antipkin Y, et al. Comparison of two inhaled tobramycin solutions in cystic fibrosis patients with chronic pseudomonas aeruginosa infection: results in different age subgroups. Journal of Cystic Fibrosis 2011;10 Suppl 1:S28. [Abstract no: 111; CFGD Register: PI249b; ]
-
- Mazurek H, Chiron R, Varoli G, Santoro D, Cicirello H, Antipkin Y. Efficacy on lung function and safety of multiple courses of tobramycin 300mg/4 ml nebuliser solution (Bramitob) in patients with cystic fibrosis and chronic Pseudomonas aeruginosa infection: results from a 48‐week extension phase. Journal of Cystic Fibrosis 2012;11 Suppl 1:S74. [Abstract no: 69; CFGD Register: PI249c; ]
-
- Mazurek H, Lenoir G, Pelikan L, Geidel C, Bolbas K, Antipkin Y, et al. Head‐to‐head comparison of two inhaled tobramycin solutions in cystic fibrosis (CF) patients with chronic pseudomonas aeruginosa (Pa) infection. Journal of Cystic Fibrosis 2011;10 Suppl 1:S28. [Abstract no: 110; CFGD Register: PI249a ; ]
McCoy 2008 {published data only}
-
- McCoy K, Retsch‐Bogart G, Gibson RL, Oermann C, Braff M, Montgomery AB. Investigation of susceptibility breakpoints for inhaled antibiotic therapies in cystic fibrosis. Pediatric Pulmonology 2010;45 Suppl 33:341. [Abstract no: 340; CFGD Register: PI212g/PI213i; ]
-
- McCoy K, Retsch‐Bogart G, Oermann C, Gibson R, Montgomery AB. Aztreonam lysine for inhalation (AZLI) for CF patients with P. aeruginosa (PA) infection. Journal of Cystic Fibrosis 2007;6 Suppl 1:S10. [CFGD Register: PI212a]
-
- McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch‐Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2008;178(9):921‐8, Online data supplement. [CFGD Register: PI212d] - PMC - PubMed
-
- McCoy KS, Retsch‐Boagrt GZ, Gibson RL, Oermann CM, McKevitt M, Montgomery AB. Efficacy of aztreonam lysine for inhalation (AZLI) in patients with cystic fibrosis and drug resistant P. aeruginosa (DRPA). Journal of Cystic Fibrosis 2009;8 Suppl 2:S28. [Abstract no: 108; CFGD Register: PI212e // PI 213e]
Mullinger 2005 {published data only}
-
- Mullinger B, Brand P, Fischer A, Haubermann S, Scheuch G, Seitz J, et al. Intra‐pulmonal deposition of two different tobramycin formulations. Journal of Cystic Fibrosis 2005;4 Suppl 1:S53.
Nasr 2004 {published data only}
-
- Nasr SZ, Gordon D, Sakmar E, Eckhardt BP, Strouse PJ. Comparison of high resolution computerized tomography (HRCT) of the chest and pulmonary function testing in evaluating the effect of tobramycin solution for inhalation in cystic fibrosis (CF) patients. European Respiratory Journal 2004;24 Suppl 48:P2403. [CFGD Register: DG2a]
-
- Nasr SZ, Gordon D, Sakmar E, Yu X, Christodoulou E, Eckhardt BP, et al. High resolution computerized tomography of the chest and pulmonary function testing in evaluating the effect of tobramycin solution for inhalation in cystic fibrosis patients. Pediatric Pulmonology 2006;41(12):1129‐37. [CFGD Register: DG2c] - PubMed
-
- Nasr SZ, Gordon D, Yu X, Sakmar E, Eckhardt BP, Strause P. Comparison of high resolution computerized tomography (HRCT) of the chest and pulmonary function testing in evaluating of the effect of tobramycin solution for inhalation (TSI) in cystic fibrosis (CF) subjects with mild lung disease. Pediatric Pulmonology 2004;38 Suppl 27:299. [CFGD Register: DG2b]
-
- Nasr SZ, Sakmar E, Christodoulou E, Eckhardt BP, Streetman DS, Strouse PJ. The use of high resolution computerized tomography (HRCT) of the chest in evaluating the effect of tobramycin solution for inhalation in cystic fibrosis lung disease. Pediatric Pulmonology 2010;45(5):440‐9. [CFGD Register: DG2e] - PubMed
-
- Nasr SZ, Sakmar E, Eckhardt BP, Strouse PJ. High resolution computerized tomography (HRCT) of the chest vs. pulmonary function testing utility in evaluating the effect of tobramycin solution for inhalation in cystic fibrosis. Pediatric Pulmonology 2008;43 Suppl 31:366. [CFGD Register: DG2d]
Nikolaizik 1996 {published data only}
-
- Nikolaizik WH, Jenni JV, Schoni MH. Bronchial constriction after nebulized tobramycin preparations and saline in patients with cystic fibrosis. European Journal of Pediatrics 1996;155:608‐11. [CFGD Register: PI144] - PubMed
Noah 2007 {published data only}
-
- Noah T, Ivins S, Abode K, Harris W, Henry M, Leigh M. Comparison of antibiotics for early pseudomonas infection in CF: interim data analysis. Pediatric Pulmonology 2007;42 Suppl 30:332. [CFGD Register: PI205a]
-
- Noah TL, Ivins SS, Abode KA, Stewart PW, Michelson PH, Harris WT, et al. Inhaled versus systemic antibiotics and airway inflammation in children with cystic fibrosis and Pseudomonas. Pediatric Pulmonology 2010;45(3):281‐90. [CFGD Register: PI205b] - PubMed
Nolan 1982 {published data only}
-
- Nolan G, McIvor P, Levison H, Fleming PC, Corey M, Gold R. Antibiotic prophylaxis in cystic fibrosis: inhaled cephaloridine as an adjunct to oral cloxacillin. Journal of Pediatrics 1982;101(4):626‐30. - PubMed
Oermann 2009 {published data only}
-
- Oermann CM, McCoy KS, Retsch‐Bogart GZ, Gibson R, McKevitt M, Montgomery B. Antibiotic susceptibility in Pseudomonas aeruginosa (PA) isolates following repeated exposure to aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis. Pediatric Pulmonology 2009;44 Suppl 32:309. [Abstract no: 278]
-
- Oermann CM, McCoy KS, Retsch‐Bogart GZ, Gibson R, McKevitt M, Montgomery B. Effect of repeated exposure to aztreonam for inhalation solution (AZLI) therapy on cystic fibrosis respiratory pathogens. Pediatric Pulmonology 2009;44 Suppl 32:335. [Abstract no:: 353]
-
- Oermann CM, McCoy KS, Retsch‐Bogart GZ, Gibson RL, Montgomery AB. Effect of multiple courses of aztreonam lysine for inhalation (AZLI) on FEV1 and weight in patients with cystic fibrosis (CF) and Pseudomonas aeruginosa (PA): analysis of 18 month data from CP‐AI‐006. Journal of Cystic Fibrosis 2009;8 Suppl 2:S28. [Abstract no: 107]
-
- Oermann CM, McCoy KS, Retsch‐Bogart GZ, Gibson RL, Quittner AL, Montgomery AB. Adherence over multiple courses of Aztreonam for inhalation (AZLI): effect on disease‐related end points in patients with cystic fibrosis (CF) and Pseudomonas aeruginosa (PA). Journal of Cystic Fibrosis 2009;8 Suppl 2:S28. [Abstract no: 109]
Poli 2005 {published data only}
-
- Poli G, Acerbi D, Pennini R, Soliani Raschini A, Bianco F, et al. Clinical pharmacology study of a new tobramycin solution for nebulisation. Journal of Cystic Fibrosis 2006;5 Suppl:S43. [CFGD Register: PI195b]
-
- Poli G, Acerbi D, Pennini R, Soliani Raschini A, Corrado M, Eichler HG, Eichler I. Clinical pharmacology study of a new tobramycin solution for nebulisation. European Respiratory Journal 2005;26 Suppl 49:729s. [CFGD Register: PI195a] - PubMed
-
- Poli G, Acerbi D, Pennini R, Soliani Raschini A, Corrado ME, Eichler HG, et al. Clinical pharmacology study of Bramitob, a tobramycin solution for nebulization, in comparison with Tobi. Paediatric Drugs 2007;9 Suppl 1:3‐9. [CFGD Register: PI195c] - PubMed
Pradal 2002 {published data only}
-
- Pradal U, Casotti V, Delmarco A, Nicolis E, Livraghi A, Conese M, et al. Effects of gentamicin on ion transport, MRNA and protein CFTR expression in patients with R1162X: a double blind placebo controlled study. Pediatric Pulmonology 2002;Suppl 24:263. [CFGD Register: BD145]
Proesmans 2008 {published data only}
-
- Proesmans M, Boulanger L, Vermeulen F, Boeck K. Eradication of recent Pseudomonas aeruginosa isolation: TOBI versus colistin/ ciprofloxacin. Journal of Cystic Fibrosis 2008;7 Suppl 2:S64. [CFGD Register: PI208a]
-
- Proesmans M, Boulanger L, Vermeulen F, Boeck K. Eradication of recent Pseudomonas aeruginosa isolation: TOBI versus colistin/ciprofloxacin. Pediatric Pulmonology 2009;44 Suppl 32:321. [Abstract no: 311; CFGD Register: PI208b]
-
- Proesmans M, Boulanger L, Vermeulen F, Boeck K. Eradication of recent pseudomonas aeruginosa infection: TOBI versus Colistineb®/ ciprofloxacin. Journal of Cystic Fibrosis 2011;10 Suppl 1:S26. [Abstract no: 102; CFGD Register: PI208c]
-
- Proesmans M, Vermeulen F, Boulanger L, Verhaegen J, Boeck K. Comparison of two treatment regimens for eradication of pseudomonas aeruginosa infection in children with cystic fibrosis. Journal of Cystic Fibrosis 2016;12(1):29‐34. [CFGD Register: PI208d] - PubMed
Ramsey 1993 {published data only}
-
- Fiel SB. Aerosol delivery of antibiotics to the lower airways of patients with cystic fibrosis. Chest 1995;107 Suppl 2:61S‐4S. [CFGD Register: PI75b] - PubMed
-
- Ramsey BW, Dorkin HL, Eisenberg JD, Gibson RL, Harwood IR, Kravitz RM, et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. New England Journal of Medicine 1993;328(24):1740‐6. [CFGD Register: PI75a] - PubMed
Ramsey 2005 {published data only}
-
- Anstead M, Lymp J, Khan U, Barbieri J, Langkamp M, Doring G, et al. Pseudomonas aeruginosa serology predicts response to treatment and re‐infection in the EPIC clinical study. Pediatric Pulmonology 2001;46 Suppl 34:303. [Abstract no: 254; CFGD Register: PI202g]
-
- Anstead M, Saiman L, Mayer‐Hamblett N, Lands LC, Kloster M, Goss CH, et al. Pulmonary exacerbations in CF patients with early lung disease. Journal of Cystic Fibrosis 2014;13(1):74‐9. [CENTRAL: 961723; CFGD Register: PI202l; CRS: 5500050000000096; EMBASE: 2013796396] - PubMed
-
- Hamblett NM, Retsch‐Bogart GZ, Treggiari M, Kronmal RA, Khan U, Williams J, et al. Safety and efficacy of anti‐pseudomonal therapy for early eradication of Pseudomonas aeruginosa: the EPIC study. Pediatric Pulmonology 2009;44 Suppl 32:183. [PI202b]
-
- Hoffman LR, Ramsey BW, Kulasekara HD, Retsch‐Bogart GZ, Wolter DJ, Pope CE, et al. Pseudomonas aeruginosa (PA) phenotypes associated with persistent early infection in CF patients in the EPIC clinical trial. Pediatric Pulmonology 2012;47 Suppl 35:317. [Abstract no: 266; CFGD Register: PI202j]
Ratjen 2006 {published data only}
-
- Ratjen F, Munck A, Campello V. Inhaled tobramycin nebuliser solution for treatment of early Pseudomonas aeruginosa infection: first results from the Elite study. Pediatric Pulmonlogy 2006;41 Suppl 29:318. [CFGD Register: PI197b]
-
- Ratjen F, Munck A, Campello V. Safety of inhaled tobramycin nebuliser solution for treatment of early pseudomonas aeruginosa infection: first results from the ELITE study. Journal of Cystic Fibrosis 2006;5 Suppl:S22. [CFGD Register: PI197a]
-
- Ratjen F, Munck A, Kho P. Short and long‐term efficacy of inhaled tobramycin in early P. aeruginosa infection: the ELITE study. Pediatric Pulmonology 2008;43 Suppl 31:319. [CFGD Register: PI197d]
-
- Ratjen F, Munck A, Kho P, Angyalosi G. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial. Thorax 2010;65(4):286‐91. [CFGD Register: PI197e] - PubMed
-
- Ratjen F, Stenglein S, Munck A. Inhaled tobramycin nebulizer solution for treatment of early Pseudomonas aeruginosa infection; the ELITE study. Journal of Cystic Fibrosis 2008;7 Suppl 2:S26. [CFGD Register: PI197c]
Retsch‐Bogart 2007 {published data only}
-
- McCoy K, Retsch‐Bogart G, Gibson RL, Oermann C, Braff M, Montgomery AB. Investigation of susceptibility breakpoints for inhaled antibiotic therapies in cystic fibrosis. Pediatric Pulmonology 2010;45 Suppl 33:341. [Abstract no: 340; CFGD Register: PI212g/PI213i; ]
-
- McCoy KS, Retsch‐Boagrt GZ, Gibson RL, Oermann CM, McKevitt M, Montgomery AB. Efficacy of aztreonam lysine for inhalation (AZLI) in patients with cystic fibrosis and drug resistant P. aeruginosa (DRPA). Journal of Cystic Fibrosis 2009;8 Suppl 2:S28. [Abstract no: 108; CFGD Register: PI213e // PI212e]
-
- McCoy KS, Retsch‐Bogart GZ, Gibson R, Oermann C, Braff MH, Montgomery AB. Relevance of established susceptibility breakpoints to clinical efficacy of inhaled antibiotic therapies in cystic fibrosis. Pediatric Pulmonology 2008;43 Suppl 31:351. [Abstract no: 418; CFGD Register: PI212b // PI213c]
-
- Plosker GL. Aztreonam lysine for inhalation solution in cystic fibrosis. Drugs 2010;70(14):1843‐55. [CFGD Register: PI213h] - PubMed
-
- Quitnner AL, Henig NR, Lewis S, Derchak PA, McCoy KS, Oermann CM, et al. Effects of chronic intermittent aztreonam for inhalation solution (AZLI) on health‐related quality of life (HRQOL) in persons with cystic fibrosis (CF) and P. aeruginosa. Pediatric Pulmonology 2011;46 Suppl 34:299. [Abstract no: 240; CFGD Register: PI213j ; ]
Retsch‐Bogart 2008 {published data only}
-
- Burns JL, Stapp J, Lofland D, AI Phase 2 Study Group. Microbiology results from a phase 2 clinical study of aztreonam lysinate for inhalation (AI): a new inhaled antibiotic to treat CF patients with Pseudomonas aeruginosa (PA). Journal of Cystic Fibrosis 2005;4 Suppl:S55. [CFGD Register: PI211a]
-
- Retsch‐Bogart GZ, Burns JL, Otto KL, Liou TG, McCoy K, Oermann C, et al. A phase 2 study of aztreonam lysine for inhalation to treat patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatric Pulmonology 2008;43(1):47‐58. [CFGD Register: PI211c] - PubMed
-
- Retsch‐Bogart GZ, Gibson RL, AI Phase 2 Study Group. A phase 2 study of aztreonam lysinate for inhalation to treat cystic fibrosis patients with Pseudomonas aeruginosa infection. American Thoracic Society International Conference; 2005 May 20‐25; San Diego, USA. 2005:A576. [CFGD Register: PI211b]
-
- Retsch‐Bogart GZ, McCoy KS, Gibson RL, Oermann CM, Braff MH, Montgomery AB. Sustained improvement in pulmonary function following a 28‐day course of 75 MG AZLI TID therapy. Pediatric Pulmonology 2008;43 Suppl 31:320. [CFGD Register: PI211d]
Rietschel 2009 {published data only}
-
- Rietschel E, Posselt HG, Heuer HE, Merkel N, Staab D. Pharmacokinetics of tobramycin (TOBI™) after 4 and 8 weeks of continuous once daily or twice daily inhalation. Journal of Cystic Fibrosis 2009;8 Suppl 2:S27. [Abstract no: 106; CFGD Register: PI232a]
-
- Rietschel E, Staab D, Merkel N, Konigsbruggen S, Posselt H. Pharmacokinetics of continuous treatment with o.d. or b.i.d inhalation of tobramycin (TOBI™) via Pari EFlow™ Rapid. Pediatric Pulmonology 2010;45 Suppl 33:320. [Abstract no: 283; CFGD Register: PI232c]
-
- Rietschel E, Staab D, Konigsbruggen S, Merkel N, Heuer HE, Posselt HG. Pharmacokinetics of continuous treatment with o.d. or b.i.d. inhalation of tobramycin (TOBI™). Journal of Cystic Fibrosis 2010;9 Suppl 1:S23. [Abstract no: 85; CFGD Register: PI232b]
-
- Koningsbruggen‐Rietschel S, Heuer HE, Merkel N, Posselt HG, Staab D, Sieder C, et al. Pharmacokinetics and safety of an 8 week continuous treatment with once‐daily versus twice‐daily inhalation of tobramycin in cystic fibrosis patients. Journal of Antimicrobial Chemotherapy 2016;71(3):711‐7. [CENTRAL: 1199846; CFGD Register: PI232d; CRS: 5500135000001729; PUBMED: 26626719] - PubMed
Rosenfeld 2006 {published data only}
-
- Rosenfeld M, Emerson J, Uh D, Anderson G, Genatossio A, McNamara S, et al. Does tobramycin accumulate in respiratory secretions with repeated aerosol administration: a pilot study. Pediatric Pulmonology 2006;41 Suppl 29:327. [CFGD Register: PI203]
Ruddy 2013 {published data only}
Sands 2014 {published data only}
-
- Sands D, Sapiejka E, Gaszczyk G, Mazurek H, for the T100 Study Group. Comparison of two tobramycin nebuliser solutions: pharmacokinetic, efficacy and safety profiles of T100 and TNS. Journal of Cystic Fibrosis 2014;13:653‐60. [CFGD Register: PI263c] - PubMed
-
- Sands D, Sapiejka E, Mazurek H, Gaszczyk G. A randomised comparison of VANTOBRA and TOBI in cystic fibrosis patients with chronic pseudomonas aeruginosa infection for pharmacokinetic and therapeutic equivalence. Journal of Cystic Fibrosis 2013;12 Suppl 1:S66. [Abstract no: 69; CFGD Register: PI263a; ]
-
- Sands D, Sapiejka E, Mazurek H, Gaszczyk G. Use of an electronic monitoring system to generate objective information on patients' adherence to taking treatments of a novel inhaled tobramycin solution. European Respiratory Journal 2013;42 Suppl 57:P1188. [Abstract no: 1497; CENTRAL: 1099909; CFGD Register: PI263d; CRS: 5500050000000417; EMBASE: 71843058]
-
- Sands D, Sapiejka E, Mazurek H, Gaszczyk G. Use of an electronic monitoring system to generate objective information on patients' adherence to taking treatments of a novel tobramycin solution (VANTOBRA). Journal of Cystic Fibrosis 2013;12 Suppl 1:S66. [Abstract no: 70; CFGD Register: PI263b; ]
Schaad 1987 {published data only}
-
- Schaad UB, Wedgwood‐Krucko J, Suter S, Kraemer R. Efficacy of inhaled amikacin as adjunct to intravenous combination therapy (ceftazidime and amikacin) in cystic fibrosis. Journal of Pediatrics 1987;111(4):599‐605. [CFGD Register: PI56] - PubMed
Schaad 1997 {published data only}
-
- Schaad UB, Wedgwood J, Reuderberg A, Kraemer R, Hampel B. Ciprofloxacin as antipseudomonal treatment in patients with cystic fibrosis. Pediatric Infectious Disease Journal 1997;16(1):106‐11. [CFGD Register: PI116; CFGD Register: PI116; ] - PubMed
Shatunov 2001 {published data only}
-
- Shatunov SM. Comparative efficacy of different methods of ceftazidime administration in children with cystic fibrosis. 11th European Respiratory Society Annual Congress; 2001 Sept 22‐26; Berlin. 2001:860. [CFGD Register: PI164]
Smith 1989 {published data only}
-
- Smith AL, Ramsey BW, Hedges DL, Hack B, Williams‐Warren J, Weber A, et al. Safety of aerosol tobramycin administration for three months to patients with cystic fibrosis. Pediatric Pulmonology 1989;7:265‐71. - PubMed
Stass 2009 {published data only}
-
- Stass H, Nagelschmitz J, Posselt H. Safety, tolerability and pharmacokinetics of ciprofloxacin dry powder for inhalation in adolescent patients with CF. Pediatric Pulmonology 2009;44 Suppl 32:302. [Abstract no: 258; CFGD Register: PI233]
Stass 2014 {published data only}
-
- Stass H, Delesen H, Nagelschmitz J, Staab D. Safety and pharmacokinetics of ciprofloxacin dry powder for inhalation in cystic fibrosis: a phase I, randomized, single‐dose, dose‐escalation study. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2015;28(2):106‐15. [CFGD Register: PI215c; DOI: 10.1089/jamp.2013.1056] - DOI - PubMed
-
- Stass H, Ludwig M, Nagelschmitz J, Stabb D. Safety and pharmacokinetics of inhaled dry powder ciprofloxacin after single and multiple inhalations in patients with cystic fibrosis. Pediatric Pulmonology 2008;43 Suppl 31:300. [CFGD Register: PI215a]
-
- Stass H, Weimann B, Nagelschmitz J, Rolinck‐Werninghaus C, Staab D. Tolerability and pharmacokinetic properties of ciprofloxacin dry powder for inhalation in patients with cystic fibrosis: a phase I, randomized, dose‐escalation study. Clinical Therapeutics 2013;35(10):1571‐81. [CFGD Register: PI215b] - PubMed
Steinkamp 1989 {published data only}
-
- Gappa M, Steinkamp G, Tummler B, Hardt H. Long‐term tobramycin aerosol therapy of chronic Pseudomonas aeruginosa infection in patients with cystic fibrosis. Scandinavian Journal of Gastroenterology 1988;23 Suppl 143:74‐6. - PubMed
-
- Steinkamp G, Tummler B, Gappa M, Albus A, Potel J, Doring G, et al. Long‐term tobramycin aerosol therapy in cystic fibrosis. Pediatric Pulmonology 1989;6:91‐8. - PubMed
Steinkamp 2006 {published data only}
-
- Steinkamp G, Schmitt‐Grohe S, Doring G, Stabb D, Schubert R, Zielen S. Once weekly azithromycin in cystic fibrosis: a double‐blind, randomised trial in patients with chronic Pseudomonas aeruginosa infection. Pediatric Pulmology 2007;42 Suppl 30:300.
-
- Steinkamp G, Schmitt‐Grohe S, Doring G, Worlitzsch D, Staab D, Schubert R, et al. Clinical and immunomodulatory effects of once weekly azithromycin treatment in cystic fibrosis patients chronically infected with Pseudomonas aeruginosa. Journal of Cystic Fibrosis 2006;5 Suppl:S25.
Stephens 1983 {published data only}
-
- Stephens D, Garey D, Isles A, Levison H, Gold R. Efficacy of inhaled tobramycin in the treatment of pulmonary exacerbations in children with cystic fibrosis. Pediatric Infectious Disease Journal 1983;2(3):209‐11. [CFGD Register: PI105] - PubMed
Stroobant 1985 {published data only}
-
- Heaf DP, Tyson S, Dinwiddie R, Matthew D. A comparison of inhaled therapies in children with cystic fibrosis. 9th International Cystic Fibrosis Congress; 1984 June 9‐15; Brighton, England. 1984:274. [CFGD Register: PI83a]
-
- Stroobant J, Heaf DP, Tyson S, Matthew DJ. Effect of inhaled azlocillin, mistabron and combination therapy in children with cystic fibrosis. 13th Annual Meeting of European Working Group for Cystic Fibrosis; 1985 Nov 3‐8; Jerusalem. 1985:47. [CFGD Register: PI83b]
-
- Stroobant J, Heaf DP, Tyson S, Matthew DJ. Effect of inhaled azlocillin, mistabron and combination therapy in children with cystic fibrosis. Pediatric Research 1985;19:1099. [CFGD Register: PI83c]
Tramper‐Stranders 2009 {published data only}
-
- Tramper‐Stranders G, Wolfs TFW, Aalderen W, Kouwenberg J, Nagelkerke A, Ent CK. Prevention of initial P. aeruginosa infection in children with cystic fibrosis: a multi‐centre double‐blind randomised controlled trial. Journal of Cystic Fibrosis 2009;8 Suppl 2:S37. [Abstract no: 148; CFGD Register: PI239a]
-
- Tramper‐Stranders GA, Wolfs TF, Haren Noman S, Aalderen WM, Nagelkerke AF, Nuijsink M, et al. Controlled trial of cycled antibiotic prophylaxis to prevent initial Pseudomonas aeruginosa infection in children with cystic fibrosis. Thorax 2010;65(10):915‐20. [CENTRAL: 761942; CFGD Register: PI239b; CRS: 5500125000000359; PUBMED: 20729233] - PubMed
Trapnell 2012 {published data only}
-
- McColley SA, Trapnell B, Kissner D, McKevitt M, Montgomery B, Rosen J, et al. Fosfomycin/tobramycin for inhalation (FTI): microbiological results of a phase 2 placebo‐controlled trial in patients with cystic fibrosis and pseudomonas aeruginosa. Pediatric Pulmonology 2010;45 Suppl 33:338. [Abstract no: 334; CFGD Register: PI247c; CFGD Register: PI247c; ]
-
- Trapnell BC, Kissner D, Montgomery AB, Newcomb T, Geller D. Fosfomycin/tobramycin for inhalation FTI): safety results of a phase 2 placebo‐controlled trial in patients with cystic fibrosis and pseudomonas aeruginosa. Pediatric Pulmonology 2010;45 Suppl 33:302. [Abstract no: 234; CFGD Register: PI247b; ]
-
- Trapnell BC, McColley SA, Kissner DG, Rolfe MW, Rosen JM, McKevitt M, et al. Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with pseudomonas airway infection. American Journal of Respiratory and Critical Care Medicine 2012;185(2):171‐8. [CFGD Register: PI247d; ] - PMC - PubMed
-
- Trapnell BC, Rolfe M, McColley S, Montgomery AB, Moorehead L, Geller D. Fosfomycon/tobramycin for inhalation (FTI): efficacy results of a phase 2 placebo‐controlled trial in patients with cystic fibrosis and pseudomonas aeruginosa. Pediatric Pulmonology 2010;45 Suppl 33:302. [Abstract no: 233; CFGD Register: PI247a; ]
Valerius 1991 {published data only}
-
- Valerius NH, Koch C, Hoiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet 1991;338(8769):725‐6. [CFGD Register: PI70b] - PubMed
-
- Valerius NH, Koch C, Hoiby N. Prevention of chronic colonization with Pseudomonas aeruginosa in patients with CF by early treatment with ciprofloxacin and colistin aerosol inhalations. Pediatric Pulmonology 1990;Suppl 5:219. [CFGD Register: PI70a]
Wainwright 2008 {published data only}
-
- Byrnes CA, Vidmar S, Cheney JL, Carlin JB, Armstrong DS, Cooper PJ, et al. Prospective evaluation of respiratory exacerbations in children with cystic fibrosis from newborn screening to 5 years of age. Thorax 2013;68(7):643‐51. [CENTRAL: 904924; CFGD Register: PE167i; CRS: 5500125000000477; PUBMED: 23345574] - PMC - PubMed
-
- Cheney J, Vidmar S, Grimwood K, Carlin JB, Wainwright C, on behalf of ACFBAL Study Group. Interim outcomes of Pseudomonas aeruginosa (Pa) eradication protocol in young children in the Australasian Cystic Fibrosis Bronchoalveolar Lavage (ACFBAL) Study. Journal of Cystic Fibrosis 2009;8 Suppl 2:S39. [Abstract no: 157; CENTRAL: 744130; CFGD Register: PE167c; CRS: 5500100000003458]
-
- Cheney J, Wainwright C, ACFBAL Study Group. Trials, tribulations and triumphs of a cystic fibrosis study ‐ a behind the scenes look at the workings of an international multi‐centre study. Journal of Cystic Fibrosis 2010;9 Suppl 1:S118. [Abstract no: 452; CENTRAL: 790042; CFGD Register: PE167g; CRS: 5500125000000092]
-
- Kidd TJ, Ramsay KA, Vidmar S, Carlin JB, Bell SC, Wainwright CE, et al. Pseudomonas aeruginosa genotypes acquired by children with cystic fibrosis by age 5‐years. Journal of Cystic Fibrosis 2015;14(3):361‐9. [CENTRAL: 1131104; CFGD Register: PE167k ; CRS: 5500135000001467; PUBMED: 25563522] - PubMed
-
- Moodie M, Lal A, Vidmar S, Armstrong DS, Byrnes CA, Carlin JB, et al. Costs of bronchoalveolar lavage‐directed therapy in the first 5 years of life for children with cystic fibrosis. Journal of Pediatrics 2014;165(3):564‐9. [CFGD Register: PE167j; CRS: 5500131000000365; JID:: 0375410; PUBMED: 24996984; SI:: ANZCTR/ACTRN12605000665639] - PubMed
Wainwright 2011 {published data only}
-
- Wainwright C, Nakamura C, Geller D, Montgomery AB. A double‐blind, multinational, randomized, placebo‐controlled trial evaluating aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis (CF), mild lung disease and P. aeruginosa. Journal of Cystic Fibrosis 2010;9 Suppl 1:S22. [Abstract no: 81; CFGD Register: PI243a]
-
- Wainwright CE, Quittner AL, Geller DE, Nakamura C, Wooldridge JL, Gibson RL, et al. Aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis, mild lung impairment, and P. aeruginosa. Journal of Cystic Fibrosis 2011;10:234‐42. [CFGD Register: PI243b; AIR‐CF4 Study Group] - PubMed
Wall 1984 {published data only}
-
- Wall MA, Steinbach S, McNamara M, Terry AB. Prophylactic inhaled antibiotics in cystic fibrosis. 25th Annual Meeting Cystic Fibrosis Club Abstracts;1984; San Jose. 1984:35.
-
- Wall MA, Terry AB, Eisenberg J, McNamara M, Cohen R. Inhaled antibiotics in cystic fibrosis. Lancet 1983;1(8337):1325. - PubMed
Wang 1984 {published data only}
-
- Wang CI, Roldan MA, Aufderheide M, Sehgal S. The effect of tobramycin aerosol to pulmonary infection from Pseudomonas aeruginosa in patients with cystic fibrosis. 25th Annual Meeting Cystic Fibrosis Club Abstracts;1984; San Jose. 1984:159.
Westerman 2003 {published data only}
-
- Westerman EM, Brun PP, Touw DJ, Frijlink HW, Heijerman HG. Effect of nebulized colistin sulphate and colistin sulphomethate on lung function in patients with cystic fibrosis: a pilot study. Journal of Cystic Fibrosis 2004;3(1):23‐8. - PubMed
-
- Westerman EM, Brun PPH, Frijlink HW, Heijerman HGM. Effect of nebulised colistin sulphate and colistin sulphomethate on lung function in patients with cystic fibrosis. Journal of Cystic Fibrosis 2003;2 Suppl 1:S55. - PubMed
Westerman 2007 {published data only}
-
- Westerman EM, Boer AH, Brun PP, Touw DJ, Roldaan AC, Frijlink HW, et al. Dry powder inhalation of colistin in cystic fibrosis patients: a single dose pilot study. Journal of Cystic Fibrosis 2007;6(4):284‐92. [CFGD Register: PI193b] - PubMed
-
- Westerman EM, Boer AH, Brun PPH, Touw DJ, Frijlink HW, Heijerman HGM. Colistin dry powder inhalation in cystic fibrosis: novel Twincer® inhaler compared to nebulization: a pilot study. Journal of Cystic Fibrosis 2005;4 Suppl 1:S64. [CFGD Register: PI193a]
Yasmin 1974 {published data only}
-
- Yasmin N, Laraya‐Cuasay LR, Mueller S, Liberi P, Braverman S, Capitanio M, et al. A critical evaluation of antibiotic aerosol in patients with cystic fibrosis. 15th Annual Meeting of Cystic Fibrosis Club; 1974. 1974. [CFGD Register: PI79]
References to studies awaiting assessment
Flume 2015 {published data only}
-
- Fischer R, Flume PA, Devanter DR, Polu K, Pecoraro M, Bhatt N, et al. Pulmonary exacerbations and changes in lung function in CF adults with P. aeruginosa treated with inhaled levofloxacin (Quinsair®) or tobramycin. Journal of Cystic Fibrosis 2016;51 Suppl 45:359. [Abstract no: 436; CENTRAL: 1262259; CRS: 5500135000001725]
-
- Flume P, Elborn JS, Polu K, Llorens L, Pecoraro ML, Bhatt N, et al. History of pulmonary exacerbations (PEx) as a predictor of response to nebulized levofloxacin compared with nebulized tobramycin. Journal of Cystic Fibrosis 2016;15 Suppl 1:S61. [Abstract no: 38; CENTRAL: 1171477; CFGD Register: PI283e; CRS: 5500135000001592]
-
- Flume P, Devanter DR, Cohen F, Fleming R, Elborn JS. Safety profile of levofloxacin inhalation solution from 3 controlled cystic fibrosis trials. Journal of Cystic Fibrosis 2015;14 Suppl 1:S87. [Abstract no: 117; CENTRAL: 1077213; CFGD Register: PI283c // PI240f // PI284c ; CRS: 5500135000001302]
-
- NCT01270347. Trial of aeroquin versus tobramycin inhalation solution (TIS) in cystic fibrosis (CF) patients [Phase 3, open‐label, randomized trial to evaluate the safety and efficacy of MP‐376 inhalation solution (Aeroquin) vs. tobramycin Iihalation solution (TIS) in stable CF patients]. clinicaltrials.gov/ct2/show/NCT01270347 05 January 2011. [CENTRAL: 1012530; CFGD Register: PI283a; CRS: 5500131000000187]
-
- Devanter D, Flume PA, Fleming R, Elborn J. How often is pulmonary exacerbation defined by ≥4 Fuchs criteria associated with antibiotic treatment?. Pediatric Pulmonology 2014;49 Suppl 38:356. [Abstract no: 388; CENTRAL: 1012531; CFGD Register: PI283b // PI284b ; CRS: 5500131000000188]
Herrmann 2017 {published data only}
-
- Herrmann G, Freitag E, Kiefer L, Bender V, Adams C, Graepler‐Mainka U, et al. Combined dry powder tobramycin and nebulized colistin versus colistin inhalation in CF patients ‐ a randomised, open label phase III clinical study. Journal of Cystic Fibrosis 2017;16 Suppl 1:S54. [CFGD Register: PI294]
Nikonova 2010 {published data only}
-
- Nikonova VS, Kashirskaya NY, Kapranov NI. Efficacy and safety of tobramycin and colistin for inhalation in children with cystic fibrosis from Moscow region. Journal of Cystic Fibrosis 2010;9 Suppl 1:S54. [Abstract no: 210; CFGD Register: PI242]
Ramsey 2017 {published data only}
-
- Ramsey BW, Retsch‐Bogart GZ, Kloster M, Buckingham R, Hamblett NM. Efficacy and safety of azithromycin for treatment of early pseudomonas in cystic fibrosis: the optimize trial. Pediatric Pulmonology 2017;52(Suppl 47):380‐1. [CFGD Register: MA31]
Additional references
Birnbaum 1998
-
- Birnbaum HG, Greenberg P, Finkelstein S, Berndt E, Otto KL, Montgomery AB, et al. Economic analysis of hospitalization and home IV anti‐pseudomonal antibiotic use in CF patients on tobramycin solution for inhalation (TOBI). Pediatric Pulmonology 1998;Suppl 17:273.
Burns 1999
-
- Burns JL, Dalfsen JM, Shawar RM, Otto KL, Garber RL, Quan JM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. Journal of Infectious Diseases 1999;179(5):1190‐6. - PubMed
CF Foundation 2017
-
- CF Foundation. About Cystic Fibrosis. www.cff.org/What‐is‐CF/About‐Cystic‐Fibrosis/ (accessed 11 December 2017).
CF Trust 2009
-
- Cystic Fibrosis Trust. Antibiotic treatment for cystic fibrosis. Consensus document; 3rd Edition; 2009 May. www.cysticfibrosis.org.uk/˜/media/documents/the‐work‐we‐do/care/consensu... (accessed prior to 08 November 2017).
CF Trust 2016
-
- Cystic Fibrosis Trust. UK Cystic Fibrosis Registry 2015 annual data report; 2016 Aug. www.cysticfibrosis.org.uk/˜/media/documents/the‐work‐we‐do/uk‐cf‐registr... (accessed prior to 08 November 2017).
CF Trust 2017
-
- CF Trust. What is cystic fibrosis ‐ how common is cystic fibrosis?. www.cysticfibrosis.org.uk/what‐is‐cystic‐fibrosis/faqs#How common is cystic fibrosis? (accessed 11 December 2017).
Dodge 2007
-
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947 ‐ 2003. European Respiratory Journal 2007;29:522‐6. - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analysis involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Emerson 2002
-
- Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson R L. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatric Pulmonology 2002;34(2):91‐100. - PubMed
Flume 2007
-
- Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Willey‐Courand DB, et al. Cystic fibrosis pulmonary guidelines. Chronic medication for maintenance of lung health. American Journal of Respiratory and Critical Care Medicine 2007;176(10):957‐69. - PubMed
GOSH 2011
-
- Great Ormond Street Hospital for Children NHS Trust. Handbook for the management of children with cystic fibrosis; 2011 Sep. www.gosh.nhs.uk/file/745/download?token=pZK‐Gnjs. Fourth edition (accessed prior to 08 Nov 2017).
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Langton Hewer 2017
Mogayzel 2013
-
- Mogayzel PJ Jr, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. American Journal of Respiratory & Critical Care Medicine 2013;187(7):680‐9. - PubMed
Montori 2005
-
- Montori VM, Devereaux PJ, Adhakiri NKJ, et al. Randomized trials stopped early for benefit. JAMA 2005;294(17):2203‐9. - PubMed
Newman 1985
-
- Newman SP. Aerosol deposition considerations in inhalational therapy. Chest 1985;88(2 Suppl):152S‐60S. - PubMed
NICE 2013
-
- National Institute for Health and Care Excellence. Colistimethate sodium and tobramycin dry powders for inhalation for treating pseudomonas lung infection in cystic fibrosis. Technology appraisal guidance TA276; 2013 Mar. www.nice.org.uk/Guidance/ta276 (accessed prior to 08 November 2017).
NICE 2017
-
- National Institute for Health and Clinical Excellence. Cystic fibrosis: diagnosis and management. nice.org.uk/guidance/ng78 2017. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenstein 1998
-
- Rosenstein BJ, Zeitlin PL. Cystic Fibrosis. Lancet 1998;351(9098):277‐82. - PubMed
Ryan 2012
Touw 1995
-
- Touw DJ, Brimicombe RW, Hodson ME, Heijerman HGM, Bakker W. Inhalation of antibiotics in cystic fibrosis. European Respiratory Journal 1995;8(9):1594‐604. - PubMed
Wark 2009
Yankaskas 2004
-
- Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: Consensus conference report. Chest 2004;125((1 suppl)):1S‐39S. - PubMed
Zemanick 2015
-
- Zemanick ET, Emerson J, Thompson V, McNamara S, Morgan W, Gibson RL, et al. Clinical outcomes after initial pseudomonas acquisition in cystic fibrosis. Pediatric Pulmonology 2015;50(1):42‐8. - PubMed
References to other published versions of this review
Ryan 1999
Ryan 2003
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous