Enteral nutritional therapy for induction of remission in Crohn's disease
- PMID: 29607496
- PMCID: PMC6494406
- DOI: 10.1002/14651858.CD000542.pub3
Enteral nutritional therapy for induction of remission in Crohn's disease
Abstract
Background: Corticosteroids are often preferred over enteral nutrition (EN) as induction therapy for Crohn's disease (CD). Prior meta-analyses suggest that corticosteroids are superior to EN for induction of remission in CD. Treatment failures in EN trials are often due to poor compliance, with dropouts frequently due to poor acceptance of a nasogastric tube and unpalatable formulations. This systematic review is an update of a previously published Cochrane review.
Objectives: To evaluate the effectiveness and safety of exclusive EN as primary therapy to induce remission in CD and to examine the importance of formula composition on effectiveness.
Search methods: We searched MEDLINE, Embase and CENTRAL from inception to 5 July 2017. We also searched references of retrieved articles and conference abstracts.
Selection criteria: Randomized controlled trials involving patients with active CD were considered for inclusion. Studies comparing one type of EN to another type of EN or conventional corticosteroids were selected for review.
Data collection and analysis: Data were extracted independently by at least two authors. The primary outcome was clinical remission. Secondary outcomes included adverse events, serious adverse events and withdrawal due to adverse events. For dichotomous outcomes, we calculated the risk ratio (RR) and 95% confidence interval (CI). A random-effects model was used to pool data. We performed intention-to-treat and per-protocol analyses for the primary outcome. Heterogeneity was explored using the Chi2 and I2 statistics. The studies were separated into two comparisons: one EN formulation compared to another EN formulation and EN compared to corticosteroids. Subgroup analyses were based on formula composition and age. Sensitivity analyses included abstract publications and poor quality studies. We used the Cochrane risk of bias tool to assess study quality. We used the GRADE criteria to assess the overall quality of the evidence supporting the primary outcome and selected secondary outcomes.
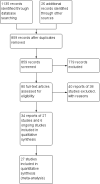
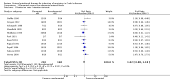
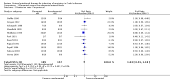
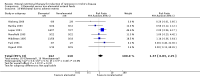
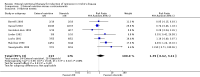
Main results: Twenty-seven studies (1,011 participants) were included. Three studies were rated as low risk of bias. Seven studies were rated as high risk of bias and 17 were rated as unclear risk of bias due to insufficient information. Seventeen trials compared different formulations of EN, 13 studies compared one or more elemental formulas to a non-elemental formula, three studies compared EN diets of similar protein composition but different fat composition, and one study compared non-elemental diets differing in glutamine enrichment. Meta-analysis of 11 trials (378 participants) demonstrated no difference in remission rates. Sixty-four per cent (134/210) of patients in the elemental group achieved remission compared to 62% (105/168) of patients in the non-elemental group (RR 1.02, 95% CI 0.88 to 1.18; GRADE very low quality). A per-protocol analysis (346 participants) produced similar results (RR 1.04, 95% CI 0.91 to 1.18). Subgroup analyses performed to evaluate the different types of elemental and non-elemental diets (elemental, semi-elemental and polymeric) showed no differences in remission rates. An analysis of 7 trials including 209 patients treated with EN formulas of differing fat content (low fat: < 20 g/1000 kCal versus high fat: > 20 g/1000 kCal) demonstrated no difference in remission rates (RR 1.03; 95% CI 0.85 to 1.26). Very low fat content (< 3 g/1000 kCal) and very low long chain triglycerides demonstrated higher remission rates than higher content EN formulas. There was no difference between elemental and non-elemental diets in adverse event rates (RR 1.00, 95% CI 0.63 to 1.60; GRADE very low quality), or withdrawals due to adverse events (RR 1.29, 95% CI 0.80 to 2.09; GRADE very low quality). Common adverse events included nausea, vomiting, diarrhea and bloating.Ten trials compared EN to steroid therapy. Meta-analysis of eight trials (223 participants) demonstrated no difference in remission rates between EN and steroids. Fifty per cent (111/223) of patients in the EN group achieved remission compared to 72% (133/186) of patients in the steroid group (RR 0.77, 95% CI 0.58 to 1.03; GRADE very low quality). Subgroup analysis by age showed a difference in remission rates for adults but not for children. In adults 45% (87/194) of EN patients achieved remission compared to 73% (116/158) of steroid patients (RR 0.65, 95% CI 0.52 to 0.82; GRADE very low quality). In children, 83% (24/29) of EN patients achieved remission compared to 61% (17/28) of steroid patients (RR 1.35, 95% CI 0.92 to 1.97; GRADE very low quality). A per-protocol analysis produced similar results (RR 0.93, 95% CI 0.75 to 1.14). The per-protocol subgroup analysis showed a difference in remission rates for both adults (RR 0.82, 95% CI 0.70 to 0.95) and children (RR 1.43, 95% CI 1.03 to 1.97). There was no difference in adverse event rates (RR 1.39, 95% CI 0.62 to 3.11; GRADE very low quality). However, patients on EN were more likely to withdraw due to adverse events than those on steroid therapy (RR 2.95, 95% CI 1.02 to 8.48; GRADE very low quality). Common adverse events reported in the EN group included heartburn, flatulence, diarrhea and vomiting, and for steroid therapy acne, moon facies, hyperglycemia, muscle weakness and hypoglycemia. The most common reason for withdrawal was inability to tolerate the EN diet.
Authors' conclusions: Very low quality evidence suggests that corticosteroid therapy may be more effective than EN for induction of clinical remission in adults with active CD. Very low quality evidence also suggests that EN may be more effective than steroids for induction of remission in children with active CD. Protein composition does not appear to influence the effectiveness of EN for the treatment of active CD. EN should be considered in pediatric CD patients or in adult patients who can comply with nasogastric tube feeding or perceive the formulations to be palatable, or when steroid side effects are not tolerated or better avoided. Further research is required to confirm the superiority of corticosteroids over EN in adults. Further research is required to confirm the benefit of EN in children. More effort from industry should be taken to develop palatable polymeric formulations that can be delivered without use of a nasogastric tube as this may lead to increased patient adherence with this therapy.
Conflict of interest statement
Neeraj Narula has no known declarations of interest to declare.
Amit Dhillon has no known declarations of interest to declare.
Dongni Zhang has no known declarations of interest to declare.
Mary Sherlock has served as an advisory board member for Abbvie and Jannsen and received travel expenses from Abbvie to attend an IBD meeting in 2015.
Melody Tondeur has no known declarations of interest to declare.
Walter Reinisch has served as a speaker, a consultant or an advisory board member for Abbott Laboratories, Abbvie, Aesca, Amgen, AM Pharma, Aptalis, Astellas, Astra Zeneca, Avaxia, Bioclinica, Biogen IDEC, Bristol‐Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Galapagos, Genentech, Grünenthal, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Robarts Clinical Trial, Schering‐Plough, Setpointmedical, Shire, Takeda, Therakos, Tigenix, UCB, Vifor, Yakult, Zyngenia, and 4SC.
Mary Zachos has served as an advisory board member for Abbvie, Janssen and Ferring.
Figures







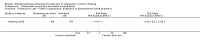

















Update of
-
Enteral nutritional therapy for induction of remission in Crohn's disease.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD000542. doi: 10.1002/14651858.CD000542.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2018 Apr 01;4:CD000542. doi: 10.1002/14651858.CD000542.pub3. PMID: 17253452 Updated.
References
References to studies included in this review
Akobeng 2000 {published data only}
-
- Akobeng AK, Miller V, Stanton J, Elbadri AM, Thomas AG. Double‐blind randomized controlled trial of glutamine‐enriched polymeric diet in the treatment of active Crohn's disease. Journal of Pediatriatric Gastroenterology and Nutrition 2000;30(1):78‐84. - PubMed
Bamba 2003 {published data only}
-
- Bamba T, Shimoyama T, Sasaki M, Tsujikawa T, Fukuda Y, Koganei K, et al. Dietary fat attenuates the benefits of an elemental diet in active Crohn's disease: a randomized, controlled trial. European Journal of Gastroenterology & Hepatology 2003;15(2):151‐7. - PubMed
Borrelli 2006 {published data only}
-
- Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn's disease: a randomized controlled open‐label trial. Clinical Gastroenterology and Hepatology 2006;4(6):744‐53. - PubMed
Gassull 2002 {published data only}
-
- Gassull A, Fernandez‐Banares F, Cabre E, Papo M, Giaffer MH, Sanchez‐Lombrana JL, et al. Fat composition is the differential factor to explain the primary therapeutic effect of enteral nutrition in Crohn's disease. Results of a double‐blind, randomized, multicenter European trial. Gastroenterology 2000;118:A117. - PMC - PubMed
-
- Gassull MA, Fernandez‐Banares F, Cabre E, Papo M, Giaffer MH, Sanchez‐Lombrana JL, et al. Fat composition may be a clue to explain the primary therapeutic effect of enteral nutrition in Crohn's disease: results of a double blind randomised multicentre European trial. Gut 2002;51(2):164‐8. - PMC - PubMed
Giaffer 1990 {published data only}
-
- Giaffer MH, North G, Holdsworth CD. Controlled trial of polymeric versus elemental diet in treatment of active Crohn's disease. Lancet 1990;335(8693):816‐9. - PubMed
Gonzalez‐Huix 1993 {published data only}
-
- Gonzalez‐Huix F, Leon R, Acero D, Esteve M, Figa M, Abad A, et al. Total enteral nutrition with polymeric diets (TENP) as primary treatment in Crohn's disease (CD). Preliminary results of a controlled trial [abstract]. Clinical Nutrition 1991;10:47.
Griffiths 2000 {published data only}
-
- Griffiths AM, Pendley F, Issenman R, Cockram D, Jacobsen K, Kelley MJ, et al. Elemental versus polymeric nutrition as primary therapy of active Crohn's disease: a multi‐centre pediatric randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2000;31(Suppl 2):S75.
Grogan 2012 {published data only}
-
- Grogan JL, Casson DH, Terry A, Burdge GC, El‐Matary W, Dalzell AM. Enteral feeding therapy for newly diagnosed pediatric Crohn's disease: A double‐blind randomized controlled trial with two years follow‐up. Inflammatory Bowel Diseases 2012;18(2):246‐53. - PubMed
Kobayashi 1998 {published data only}
-
- Kobayashi K, Katsumata T, Yokoyama K, Takahashi H, Igarashi M, Saigenji K. A randomized controlled study of total parenteral nutrition and enteral nutrition by elemental and polymeric diet as primary therapy in active phase of Crohn's disease. Nippon Shokakibyo Gakkai Zasshi 1998;95(11):1212‐21. - PubMed
Leiper 2001 {published data only}
Lindor 1992 {published data only}
-
- Lindor KD, Fleming CR, Burnes JU, Nelson JK, Ilstrup DM. A randomized prospective trial comparing a defined formula diet, corticosteroids, and a defined formula diet plus corticosteroids in active Crohn's disease. Mayo Clinic Proceedings 1992;67(4):328‐33. - PubMed
Lochs 1991 {published data only}
-
- Lochs H, Steinhardt HJ, Klaus‐Wentz B, Zeitz M, Vogelsang H, Sommer H, et al. Comparison of enteral nutrition and drug treatment in active Crohn's disease. Results of the European Cooperative Crohn's Disease Study. IV. Gastroenterology 1991;101(4):881‐8. - PubMed
Malchow 1990 {published data only}
-
- Malchow H, Steinhardt HJ, Lorenz‐Meyer H, Strohm WD, Rasmussen S, Sommer H, et al. Feasibility and effectiveness of a defined‐formula diet regimen in treating active Crohn's disease. European Cooperative Crohn's Disease Study III. Scandinavian Journal of Gastroenterology 1990;25(3):235‐44. - PubMed
Mansfield 1995 {published data only}
Mantzaris 1996 {published data only}
-
- Mantzaris GJ, Archavlis E, Amberiadis P, Kourtessas D, Triantafullou G. A Prospective Randomized Trial in Active Crohn's Disease Comparing prednisolone, a Polymeric Diet and a Polymeric Diet Plus Prednisolon [abstract]. Gut 1996;39 Suppl 3:A166.
-
- Mantzaris GJ, Archavlis E, Amperiadis P, Kourtessas D, Triantafyllou G. A randomized prospective trial in active Crohn's disease comparing a polymeric diet, prednisolone and a polymeric diet plus prednisolone. Gastroenterology 1996;110(4):A955.
Middleton 1995 {published data only}
-
- Middleton SJ, Rucker JT, Kirby GA, Riordan AM, Hunter JO. Long‐chain triglycerides reduce the efficacy of enteral feeds in patients with active Crohn's disease. Clinical Nutrition 1995;14(4):229‐36. - PubMed
Park 1991 {published data only}
-
- Park RH, Galloway A, Danesh B, Russell RI. Double blind trial comparing elemental and polymeric diet as primary therapy for active Crohn's disease [abstract]. Clinical Nutrition 1989;8:79.
-
- Park RH, Galloway A, Danesh B, Russell RI. Double blind trial comparing elemental and polymeric diet as primary treatment for active Crohns`s disease [abstract]. Gut 1989;30 Suppl 10:A1453.
-
- Park RH, Galloway A, Danesh BJ, Russell RI. Double‐blind controlled trial of elemental and polymeric diets as primary therapy in active Crohn's disease. European Journal of Gastroenterology and Hepatology 1991;3(6):483‐9.
Pigneur 2014 {published data only}
-
- Pigneur B, Garnier‐Lengline H, Lepage P, Schmitz J, Goulet O, Dore J. Effect of exclusive enteral nutrition on the course of CD and intestinal microbiota. Journal of Crohn's and Colitis 2014;8:S433.
Raouf 1991 {published data only}
Rigaud 1991 {published data only}
Royall 1994 {published data only}
Sakurai 2002 {published data only}
-
- Sakurai T, Matsui T, Yao T, Takagi Y, Hirai F, Aoyagi K, et al. Short‐term efficacy of enteral nutrition in the treatment of active Crohn's disease: a randomized, controlled trial comparing nutrient formulas. JPEN Journal of Parenteral and Enteral Nutrition 2002;26(2):98‐103. - PubMed
Saverymuttu 1985 {published data only}
Seidman 1991 {published data only}
-
- Seidman EG, Lohoues MJ, Turgeon J, Bouthillier L, Morin CL. Elemental diet versus prednisone as initial therapy in Crohn's disease: early and long‐term results. Gastroenterology 1991;100:150A.
Seidman 2003 {published data only}
-
- Seidman EG, Bouthillier L, Thibault L, Lepage G, Chartre M, Deslandres C, et al. Clinical & nutritional parameters in Crohn's disease patients treated with either an n‐3 PUFA‐enriched semi‐elemental or a polymeric diet. Results of a randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2003;37(3):393.
Terrin 2002 {published data only}
-
- Terrin G, Canani RB, Ambrosini A, Viola F, Mesquita MB, Nardo G, et al. A semielemental diet (Pregomin) as primary therapy for inducing remission in children with active Crohn's disease. Italian Journal of Pediatrics 2002;28(5):401‐5.
Verma 2000 {published data only}
-
- Verma S, Brown S, Giaffer MH. Elemental versus polymeric diet in treatment of active Crohn's disease: A double‐blind randomised trial. Gut 1997;41(Suppl 3):A226. - PubMed
-
- Verma S, Brown S, Giaffer MH. Randomised double‐blind trial of polymeric versus elemental diet in treatment of active Crohn's disease. Gut 1997;40(Suppl 1):A19. - PubMed
-
- Verma S, Brown S, Kirkwood B, Giaffer MH. Polymeric versus elemental diet as primary treatment in active Crohn's disease: a randomized, double‐blind trial. American Journal of Gastroenterology 2000;95(3):735‐9. - PubMed
References to studies excluded from this review
Afzal 2005a {published data only}
-
- Afzal NA, Davies S, Paintin M, Arnaud‐Battandier F, Walker‐Smith JA, Murch S, et al. Colonic Crohn's disease in children does not respond well to treatment with enteral nutrition if the ileum is not involved. Digestive Diseases and Sciences 2005;50(8):1471‐5. - PubMed
Akobeng 2002 {published data only}
-
- Akobeng AI, Clayton PE, Miller V, Hall CM, Thomas AG. Low serum concentrations of insulin‐like growth factor‐I in children with active Crohn disease: effect of enteral nutritional support and glutamine supplementation. Scandinavian Journal of Gastroenterology 2002;37:1422‐7. - PubMed
Berni Canani 2006 {published data only}
-
- Berni Canani R, Terrin G, Borrelli O, Romano MT, Manguso F, Coruzzo A, et al. Short‐ and long‐term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn's disease. Digestive and Liver Disease 2006;38(6):381‐7. - PubMed
Buchanan 2009 {published data only}
-
- Buchanan E, Gaunt WW, Cardigan T, Garrick V, McGrogan P, Russell RK. The use of exclusive enteral nutrition for induction of remission in children with Crohn's disease demonstrates that disease phenotype does not influence clinical remission. Alimentary Pharmacology & Therapeutics 2009;30(5):501‐7. - PubMed
Chiang 2009 {published data only}
-
- Chiang NYZ, Haslinger T, Richman E, Rhodes JM, Leiper K. Efficacy of enteral feeding with modulen in adults with Crohn's disease: A retrospective study. Gut 2009;58:A97.
Day 2006 {published data only}
-
- Day AS, Whitten KE, Lemberg DA, Clarkson C, Vitug‐Sales M, Jackson R, et al. Exclusive enteral feeding as primary therapy for Crohn's disease in Australian children and adolescents: A feasible and effective approach. Journal of Gastroenterology and Hepatology 2006;21(10):1609‐14. - PubMed
Engelman 1993 {published data only}
-
- Engelman JL, Black L, Murphy GM, Sladen GE. Comparison of a semi‐elemental diet (peptamen) with prednisolone in the primary treatment of active ileal Crohn's disease. Gastroenterology 1993;104:697A.
Gassull 2000 {published data only}
-
- Gassull A, Fernandez‐Banares F, Cabre E, Papo M, Giaffer MH, Sanchez‐Lombrana JL, et al. Fat composition is the differential factor to explain the primary therapeutic effect of enteral nutrition in Crohn's disease. Results of a double‐blind, randomized, multicenter European trial. Gastroenterology 2000;118:A117. - PMC - PubMed
Giffin 2014 {published data only}
-
- Giffin N, Grant A, Mahdi G, Rashid M, Otley AR. Exclusive enteral nutrition optimizes growth outcomes versus corticosteroid therapy for pediatric Crohn's disease. Gastroenterology 2014;5(Suppl 1):S‐217.
Goncalves 2014 {published data only}
-
- Goncalves JP, Quaresma L, Rego H, Ratola A, Tavares M, Trindade E, et al. Steroids may reduce the benefit of exclusive enteral feeding in paediatric Crohn's disease. Journal of Crohn's and Colitis 2014;8:S207.
Gorard 1993 {published data only}
-
- Hunt JB, Payne‐James JJ. A randomised controlled trial of elemental diet versus prednisolone in treatment of new and recurrent Crohn's disease [abstract]. Clinical Science 1989;77 Suppl 2:26p.
-
- Hunt JB, Payne‐James JJ, Palmer KR, Kumar PK, Clark M L, Farthing MJ. A randomised controlled trial of elemental diet versus prednisolone in the treatment of new & recurrent Crohns disease [abstract]. Clinical Nutrition 1989;8:80.
Grover 2012 {published data only}
-
- Grover Z, Muir R, Lewindon P. A prospective study of new cases of paediatric Crohn's disease treated with exclusive enteral nutrition: Early clinical, biochemical, mucosal and transmural outcomes. Gastroenterology 2012;142(5 Suppl. 1):S76‐7.
Gunasekera 2012 {published data only}
-
- Gunasekera AV, Rajendran N, Mendall MA, Kumar D. An igg4 specific food exclusion diet improves both clinical disease activity and quality of life in Crohn's disease; a randomised sham diet controlled study. Gastroenterology 2012;1:S567.
Hirakawa 1993 {published data only}
-
- Hirakawa H, Fukuda Y, Tanida N, Hosomi M, Shimoyama T. Home elemental enteral hyperalimentation (HEEH) for the maintenance of remission in patients with Crohn's disease. Gastroenterologia Japonica 1993;28(3):379‐84. - PubMed
Johnson 2006 {published data only}
Jones 1987 {published data only}
-
- Jones VA. Comparison of total parenteral nutrition and elemental diet in induction of remission of Crohn's disease. Long‐term maintenance of remission by personalized food exclusion diets. Digestive Diseases and Sciences 1987;32(12 Supplement):100S‐7S. - PubMed
Klerkus 2013 {published data only}
-
- Klerkus J, Szymanska S, Szczepanski M, Wiernicka A, Szymanska E, Matuszczyk M, et al. The efficacy of total enteral nutrition in inducing remission and improving nutritional status in children with moderate to severe Crohn's disease. Przegla̜d Gastroenterologiczny 2013;8(1):57‐61.
Levine 2014 {published data only}
-
- Levine A, Turner D, Gik TP, Dias JA, Veres G, Shaoul R, et al. Comparison of outcomes parameters for induction of remission in new onset pediatric Crohn's disease: Evaluation of the Porto IBD group "Growth relapse and outcomes with therapy" (GROWTH‐CD) study. Inflammatory Bowel Diseases 2014;20:278‐85. - PubMed
Ludvigsson 2004 {published data only}
-
- Ludvigsson JF, Krantz M, Bodin L, Stenhammar L, Lindquist B. Elemental versus polymeric enteral nutrition in paediatric Crohn's disease: a multicentre randomized controlled trial. Acta Paediatrica 2004;93(3):327‐35. - PubMed
Middleton 1991 {published data only}
-
- Middleton SJ, Riordan AM, Hunter JO. Comparison of elemental and peptide‐based diets in the treatment of acute Crohn's disease. Italian Journal of Gastroenterology 1991;23:609.
Munkholm Larsen 1989 {published data only}
-
- Munkholm Larsen P, Rasmussen D, Ronn B, Munck O, Elmgreen J, Binder V. Elemental diet: a therapeutic approach in chronic inflammatory bowel disease. Journal of Internal Medicine 1989;225(5):325‐31. - PubMed
Navas Lopez 2008 {published data only}
-
- Navas Lopez VM, Blasco Alonso J, Sierra Salinas C, Barco Galvez A, Vicioso Recio MI. Efficacy of exclusive enteral feeding as primary therapy for paediatric Crohn's disease. Anales de Pediatria 2008;69(6):506‐14. - PubMed
O'Morain 1984 {published data only}
Otley 2015 {published data only}
Papadopoulou 1995 {published data only}
-
- Papadopoulou A, Rawashdeh MO, Brown GA, McNeish AS, Booth IW. Remission following an elemental diet or prednisolone in Crohn's disease. Acta Paediatrica 1995;84(1):79‐83. - PubMed
Rabast 1986 {published data only}
-
- Rabast U, Heskamp R. Adjuvant therapy with elemental diet in chronic inflammatory intestinal diseases. Comparative study. Deutsche Medizinische Wochenschrift 1986;111(8):293‐7. - PubMed
Riordan 1993 {published data only}
-
- Riordan AM, Hunter JO, Cowan RE, Crampton J R, Davidson AR, Dickinson RJ. Treatment of active Crohn's disease by exclusion diet: East Anglian multicentre controlled trial. Lancet 1993;342:1131‐4. - PubMed
Ruuska 1994 {published data only}
-
- Ruuska T, Savilahti E, Maki M, Ormala T, Visakorpi JK. Exclusive whole protein enteral diet versus prednisolone in the treatment of acute Crohn's disease in children. Journal of Pediatric Gastroenterology and Nutrition 1994;19(2):175‐80. - PubMed
Sanderson 1987 {published data only}
Seidman 1993 {published data only}
-
- Seidman EG, Grifftiths AM, Jones A, Issenman R. Semi‐elemental diet versus prednisone in pediatric Crohn's disease. Gastroenterology 1993;104:A778.
Sigall‐Boneh 2014 {published data only}
-
- Sigall‐Boheh R, Pfeffer‐Gik T, Segal I, Zangen T, Boaz M, Levine A. Partial enteral nutrition with a Crohn's disease exclusion diet is effective for induction of remission in children and young adults with Crohn's disease. Inflammatory Bowel Diseases 2014;20:1353‐60. - PubMed
Sokulmez 2014 {published data only}
-
- Sökülmez P, Demirba AE, Arslan P, Diibeyaz S. Effects of enteral nutritional support on malnourished patients with inflammatory bowel disease by subjective global assessment. Turkish Journal of Gastroenterology 2014;25(5):493‐507. - PubMed
Soo 2013 {published data only}
-
- Soo J, Malik BA, Turner JM, Persad R, Wine E, Siminoski K, et al. Use of exclusive enteral nutrition is just as effective as corticosteroids in newly diagnosed pediatric Crohn's disease. Digestive Diseases and Sciences 2013;58:3584‐91. - PubMed
Thomas 1993 {published data only}
-
- Thomas AG, Taylor F, Miller V. Dietary intake and nutritional treatment in childhood Crohn's disease. Journal of Pediatric Gastroenterology and Nutrition 1993;17(1):75‐81. - PubMed
Ueki 1994 {published data only}
-
- Ueki M, Matsui T, Yamada M, Sakurai T, Yao T, Okada M, et al. Randomized controlled trial of amino acid based diet versus oligopeptide based diet in enteral nutritional therapy of active Crohn's disease. Nippon Shokakibyo Gakkai Zasshi 1994;91(9):1415‐25. - PubMed
Watanabe 2010 {published data only}
-
- Watanabe O, Ando T, Ishiguro K, Takahashi H, Ishikawa D, Miyake N, et al. Enteral nutrition decreases hospitalization rate in patients with Crohn's disease. Journal of Gastroenterology and Hepatology 2010;25(Suppl 1):S134‐7. - PubMed
Zoli 1996 {published data only}
-
- Zoli G, Care M, Falco F, Parazza M, Spano C, Gasbarrini G. Effect of oral elemental diet on nutritional status, intestinal permeability and disease activity in Crohn's patients. Gastroenterology 1996;110(4):A1054.
Zoli 1997 {published data only}
-
- Zoli G, Care M, Parazza M, Spano C, Biagi PL, Bernardi M, et al. A randomized controlled study comparing elemental diet and steroid treatment in Crohn's disease. Alimentary Pharmacology and Therapeutics 1997;11(4):735‐40. - PubMed
References to ongoing studies
NCT00265772 {published data only}
-
- NCT00265772. Phase IV Study Comparing a Nutritional Anti‐Inflammatory Treatment to Steroids for Pediatric Crohn's Disease ‐ the Molecular Basis. clinicaltrials.gov/show/NCT00265772 (accessed 3 August 2017).
NCT01728870 {published data only}
-
- NCT01728870. Comparison of Partial Enteral Nutririon (Modulen) With a Unique Diet to Exclusive Enteral Nutrition (Modulen) for the Treatment of Pediatric Crohn's Disease. A Prospective Randomized Controlled Trial. clinicaltrials.gov/show/NCT01728870 (accessed 3 August 2017).
NCT02056418 {published data only}
-
- NCT02056418. A Randomized, Controlled, Single‐blind Study of Effects of Enteral Nutrition and Corticosteroid on Intestinal Flora in Induction Remission of Crohn Disease in Adult. clinicaltrials.gov/show/NCT02056418 (accessed 23 July 2017).
NCT02231814 {published data only}
-
- NCT02231814. Pilot Study of Partial Enteral Nutrition With a Unique Diet for the Treatment of Adult Patients With Crohn's Disease. clinicaltrials.gov/show/NCT02231814 (accessed 3 August 2017).
NCT02843100 {published data only}
-
- NCT02843100. Diet for Induction and Maintenance of Remission and Re‐biosis in Crohn's Disease. clinicaltrials.gov/show/NCT02843100 (accessed 3 August 2017).
NCT03176875 {published data only}
-
- NCT03176875. Comparison of Partial and Exclusive Enteral Nutrition in the Treatment of Active Childhood‐onset Crohn's Disease. clinicaltrials.gov/show/NCT03176875 accessed 3 bAugust 2017.
Additional references
Afzal 2004
-
- Afzal NA, Zaag‐Loonen HJ, Arnaud‐Battandier F, Davies S, Murch S, Derkx B, et al. Improvement in quality of life of children with acute Crohn's disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Alimententary Pharmacology & Therapeutics 2004;20(2):167‐72. - PubMed
Afzal 2005b
-
- Afzal NA, Fagbemi A, Arnaud‐Battandier F, Paintin M, Thomson M, Walker‐Smith JA, et al. Enteral nutrition treats children with chronic Crohn's disease more effectively if the ileum is also involved. Archives of Disease in Childhood 2005;86(Suppl 1):A22.
Fell 2000
-
- Fell JM, Paintin M, Arnaud‐Battandier F, Beattie RM, Hollis A, Kitching P, et al. Mucosal healing and a fall in mucosal pro‐inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn's disease. Alimentary & Pharmacologic Therapeutics 2000;14(3):281‐9. - PubMed
Fernandez‐Banares 1995
-
- Fernandez‐Banares F, Cabre E, Esteve‐Comas M, Gassull MA. How effective is enteral nutrition in inducing clinical remission in active Crohn's disease? A meta‐analysis of the randomized clinical trials. JPEN Journal of Parenteral and Enteral Nutrition 1995;19(5):356‐64. - PubMed
Griffiths 1995
-
- Griffiths AM, Ohlsson A, Sherman PM, Sutherland LR. Meta‐analysis of enteral nutrition as a primary treatment of active Crohn's disease. Gastroenterology 1995;108(4):1056‐67. - PubMed
Guyatt 2008
Heuschkel 2000
-
- Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral nutrition and corticosteroids in the treatment of acute Crohn's disease in children. Journal of Pediatric Gastroenterology and Nutrition 2000;31(1):8‐15. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kansal 2013
Knight 2005
-
- Knight C, El‐Matary W, Spray C, Sandhu BK. Long‐term outcome of nutritional therapy in paediatric Crohn's disease. Clinical Nutrition 2005;24(5):775‐9. - PubMed
Landi 1992
-
- Landi B, Ahn TN, Cortot A, Soule JC, Rene E, Gendre JP, et al. Endoscopic monitoring of Crohn's disease treatment: a prospective, randomized clinical trial. Gastroenterology 1992;102(5):1647‐53. - PubMed
Levine 2003
-
- Levine A, Milo T, Buller H, Markowitz J. Consensus and controversy in the management of pediatric Crohn disease: an international survey. Journal of Pediatric Gastroenterology and Nutrition 2003;36(4):464‐9. - PubMed
Messori 1996
-
- Messori A, Trallori G, D'Albrasio G, Milla M, Vannozzi G, Pacini F. Defined‐formula diets versus steroids in the treatment of active Crohn's disease: a meta‐analysis. Scandinavian Journal of Gastroenterology 1996;31(3):267‐72. - PubMed
Modigliani 1990
-
- Modigliani R, Mary JY, Simon JF, Cortot A, Soule JC, Gendre JP, et al. Clinical, biological and endoscopic picture of attacks of Crohn's disease. Evolution on prednisolone. Gastroenterology 1990;98(4):811‐8. - PubMed
Rubio 2011
-
- Rubio A, Pigneur B, Garnier‐Lengline H, Talbotec C, Schmitz J, Canioni D, et al. The efficacy of exclusive nutritional therapy in paediatric Crohn's disease, comparing fractionated oral vs. continuous enteral feeding. Alimentary Pharmacology & Therapeutics 2011;33(12):1332‐9. - PubMed
Ruemmele 2014
-
- Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. Journal of Crohn's & Colitis 2014;8(10):1179‐207. - PubMed
Sandborn 2002
-
- Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Lofberg R, Modigliani R, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology 2002;122(2):512‐30. - PubMed
Sanderson 2005
-
- Sanderson IR, Croft NM. The anti‐inflammatory effects of enteral nutrition. JPEN Journal of Parenteral and Enteral Nutrition 2005;29(4 Suppl):S134‐8. - PubMed
Schunemann 2011
-
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Takagi 2006
-
- Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M, et al. Effectiveness of an 'half elemental diet' as maintenance therapy for Crohn's disease: A randomized‐controlled trial. Alimentary & Pharmacology Therapeutics 2006;24(9):1333‐40. - PubMed
Voitk 1973
-
- Voitk AJ, Echave V, Feller JH, Brown RA, Gurd FN. Experience with elemental diet in the treatment of inflammatory bowel disease. Is this a primary therapy?. Archives of Surgery 1973;107(2):329‐33. - PubMed
Wilschanski 1996
Yamamoto 2007
-
- Yamamoto T, Nakahigashi M, Saniabadi AR, Iwata T, Maruyama Y, Umegae S, et al. Impacts of long‐term enteral nutrition on clinical and endoscopic disease activities and mucosal cytokines during remission in patients with Crohn's disease: A prospective study. Inflammatory Bowel Disease 2007;13(12):1493‐1501. - PubMed
References to other published versions of this review
Zachos 2001
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

