Ambient ionisation mass spectrometry for in situ analysis of intact proteins
- PMID: 29607564
- PMCID: PMC6001466
- DOI: 10.1002/jms.4087
Ambient ionisation mass spectrometry for in situ analysis of intact proteins
Abstract
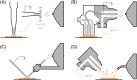
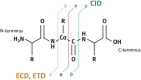
Ambient surface mass spectrometry is an emerging field which shows great promise for the analysis of biomolecules directly from their biological substrate. In this article, we describe ambient ionisation mass spectrometry techniques for the in situ analysis of intact proteins. As a broad approach, the analysis of intact proteins offers unique advantages for the determination of primary sequence variations and posttranslational modifications, as well as interrogation of tertiary and quaternary structure and protein-protein/ligand interactions. In situ analysis of intact proteins offers the potential to couple these advantages with information relating to their biological environment, for example, their spatial distributions within healthy and diseased tissues. Here, we describe the techniques most commonly applied to in situ protein analysis (liquid extraction surface analysis, continuous flow liquid microjunction surface sampling, nano desorption electrospray ionisation, and desorption electrospray ionisation), their advantages, and limitations and describe their applications to date. We also discuss the incorporation of ion mobility spectrometry techniques (high field asymmetric waveform ion mobility spectrometry and travelling wave ion mobility spectrometry) into ambient workflows. Finally, future directions for the field are discussed.
Keywords: DESI; Flowprobe; LESA; ambient mass spectrometry; intact proteins; ion mobility spectrometry; nanoDESI; surface sampling.
© 2018 The Authors. Journal of Mass Spectrometry Published by John Wiley & Sons Ltd.
Figures





Similar articles
-
Optimised Desorption Electrospray Ionisation Mass Spectrometry Imaging (DESI-MSI) for the Analysis of Proteins/Peptides Directly from Tissue Sections on a Travelling Wave Ion Mobility Q-ToF.J Am Soc Mass Spectrom. 2018 Dec;29(12):2456-2466. doi: 10.1007/s13361-018-2049-0. Epub 2018 Aug 30. J Am Soc Mass Spectrom. 2018. PMID: 30168053 Free PMC article.
-
Ambient surface mass spectrometry-ion mobility spectrometry of intact proteins.Curr Opin Chem Biol. 2018 Feb;42:67-75. doi: 10.1016/j.cbpa.2017.11.002. Epub 2018 Jan 16. Curr Opin Chem Biol. 2018. PMID: 29166625 Review.
-
Integrating ion mobility and imaging mass spectrometry for comprehensive analysis of biological tissues: A brief review and perspective.J Mass Spectrom. 2020 Dec;55(12):e4614. doi: 10.1002/jms.4614. Epub 2020 Sep 21. J Mass Spectrom. 2020. PMID: 32955134 Free PMC article.
-
Liquid Extraction Surface Analysis (LESA) High-Field Asymmetric Waveform Ion Mobility Spectrometry (FAIMS) Mass Spectrometry for In Situ Analysis of Intact Proteins.Methods Mol Biol. 2020;2084:191-201. doi: 10.1007/978-1-0716-0030-6_12. Methods Mol Biol. 2020. PMID: 31729662
-
Surface analysis of lipids by mass spectrometry: more than just imaging.Prog Lipid Res. 2013 Oct;52(4):329-53. doi: 10.1016/j.plipres.2013.04.005. Epub 2013 Apr 24. Prog Lipid Res. 2013. PMID: 23623802 Review.
Cited by
-
Protein identification by 3D OrbiSIMS to facilitate in situ imaging and depth profiling.Nat Commun. 2020 Nov 17;11(1):5832. doi: 10.1038/s41467-020-19445-x. Nat Commun. 2020. PMID: 33203841 Free PMC article.
-
Paleoproteomics.Chem Rev. 2022 Aug 24;122(16):13401-13446. doi: 10.1021/acs.chemrev.1c00703. Epub 2022 Jul 15. Chem Rev. 2022. PMID: 35839101 Free PMC article. Review.
-
Mass Spectrometry Analysis of Intact Proteins from Crude Samples.Anal Chem. 2020 Oct 6;92(19):12741-12749. doi: 10.1021/acs.analchem.0c02162. Epub 2020 Sep 22. Anal Chem. 2020. PMID: 32897050 Free PMC article.
-
In situ mass spectrometry analysis of intact proteins and protein complexes from biological substrates.Biochem Soc Trans. 2020 Feb 28;48(1):317-326. doi: 10.1042/BST20190793. Biochem Soc Trans. 2020. PMID: 32010951 Free PMC article. Review.
-
High-Performance Molecular Imaging with MALDI Trapped Ion-Mobility Time-of-Flight (timsTOF) Mass Spectrometry.Anal Chem. 2019 Nov 19;91(22):14552-14560. doi: 10.1021/acs.analchem.9b03612. Epub 2019 Oct 28. Anal Chem. 2019. PMID: 31593446 Free PMC article.
References
-
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass‐spectrometry of large biomolecules. Science. 1989;246(4926):64‐71. - PubMed
-
- Takyi‐Williams J, Liu C‐F, Tang K. Ambient ionization MS for bioanalysis: Recent developments and challenges. Bioanalysis. 2015;7(15):1901‐1923. - PubMed
-
- Huang MZ, Cheng SC, Cho YT, Shiea J. Ambient ionization mass spectrometry: A tutorial. Anal Chim Acta. 2011;702(1):1‐15. - PubMed
-
- Kertesz V, Van Berkel GJ. Fully automated liquid extraction‐based surface sampling and ionization using a chip‐based robotic nanoelectrospray platform. J Mass Spectrom. 2010;45(3):252‐260. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

