STING agonists enable antiviral cross-talk between human cells and confer protection against genital herpes in mice
- PMID: 29608601
- PMCID: PMC5897032
- DOI: 10.1371/journal.ppat.1006976
STING agonists enable antiviral cross-talk between human cells and confer protection against genital herpes in mice
Abstract
In recent years, there has been an increasing interest in immunomodulatory therapy as a means to treat various conditions, including infectious diseases. For instance, Toll-like receptor (TLR) agonists have been evaluated for treatment of genital herpes. However, although the TLR7 agonist imiquimod was shown to have antiviral activity in individual patients, no significant effects were observed in clinical trials, and the compound also exhibited significant side effects, including local inflammation. Cytosolic DNA is detected by the enzyme cyclic GMP-AMP (2'3'-cGAMP) synthase (cGAS) to stimulate antiviral pathways, mainly through induction of type I interferon (IFN)s. cGAS is activated upon DNA binding to produce the cyclic dinucleotide (CDN) 2'3'-cGAMP, which in turn binds and activates the adaptor protein Stimulator of interferon genes (STING), thus triggering type I IFN expression. In contrast to TLRs, STING is expressed broadly, including in epithelial cells. Here we report that natural and non-natural STING agonists strongly induce type I IFNs in human cells and in mice in vivo, without stimulating significant inflammatory gene expression. Systemic treatment with 2'3'-cGAMP reduced genital herpes simplex virus (HSV) 2 replication and improved the clinical outcome of infection. More importantly, local application of CDNs at the genital epithelial surface gave rise to local IFN activity, but only limited systemic responses, and this treatment conferred total protection against disease in both immunocompetent and immunocompromised mice. In direct comparison between CDNs and TLR agonists, only CDNs acted directly on epithelial cells, hence allowing a more rapid and IFN-focused immune response in the vaginal epithelium. Thus, specific activation of the STING pathway in the vagina evokes induction of the IFN system but limited inflammatory responses to allow control of HSV2 infections in vivo.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: Invivogen has partly funded this work and has a financial interest in Sting agonists. CB, TL, and EP are employees of InvivoGen. This does not alter our adherence to all PLOS Pathogens policies on sharing data and materials.
Figures


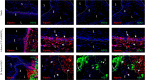
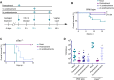
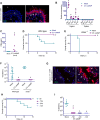
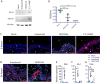
References
-
- D΄Ambrosio Roberta, Degasperi Elisabetta, Colombo Massimo, Aghemo Alessio, Direct-acting antivirals: the endgame for hepatitis C?, Current Opinion in Virology, Volume 24, 2017, Pages 31–37, ISSN 1879-6257, doi: 10.1016/j.coviro.2017.03.017 - DOI - PubMed
-
- Das K, Arnold E. HIV-1 reverse transcriptase and antiviral drug resistance. Part 1. Current Opinion in Virology. 2013;3:111–8. doi: 10.1016/j.coviro.2013.03.012 . - DOI - PMC - PubMed
-
- Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370:2127–37. doi: 10.1016/S0140-6736(07)61908-4 . - DOI - PubMed
-
- Sandgren KJ, Bertram K, Cunningham AL. Understanding natural herpes simplex virus immunity to inform next-generation vaccine design. Clinical & Translational Immunology. 2016;5:e94 doi: 10.1038/cti.2016.44 . - DOI - PMC - PubMed
-
- Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bulletin of the World Health Organization. 2008;86:805–12. doi: 10.2471/BLT.07.046128 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

