Long-term Impact of Oral Azithromycin Taken by Gambian Women During Labor on Prevalence and Antibiotic Susceptibility of Streptococcus pneumoniae and Staphylococcus aureus in Their Infants: Follow-up of a Randomized Clinical Trial
- PMID: 29608659
- PMCID: PMC6160601
- DOI: 10.1093/cid/ciy254
Long-term Impact of Oral Azithromycin Taken by Gambian Women During Labor on Prevalence and Antibiotic Susceptibility of Streptococcus pneumoniae and Staphylococcus aureus in Their Infants: Follow-up of a Randomized Clinical Trial
Erratum in
-
Erratum.Clin Infect Dis. 2018 Sep 14;67(7):1150. doi: 10.1093/cid/ciy428. Clin Infect Dis. 2018. PMID: 30124782 Free PMC article. No abstract available.
Abstract
Background: Oral azithromycin given to women in labor decreases maternal and neonatal bacterial carriage but increases azithromycin-resistant bacteria during at least 4 weeks following the intervention. We assessed the prevalence of bacterial carriage and azithromycin resistance 12 months after treatment among study infants.
Methods: Nasopharyngeal swabs (NPSs) were collected between November 2014 and May 2015 from children aged 11-13 months whose mothers had received azithromycin or placebo during labor. Streptococcus pneumoniae and Staphylococcus aureus were isolated using conventional microbiological methods. Antibiotic susceptibility was determined by disk diffusion and confirmed by Etest or VITEK-2.
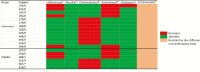
Results: NPSs were collected from 461 children. The prevalence of S. pneumoniae and S. aureus was similar between children from the azithromycin and placebo arms (85.0% vs 82.1%; odds ratio [OR], 1.23 [95% confidence interval {CI}, .73-2.08] for S. pneumoniae and 21.7% vs 21.3%; OR, 1.02 [95% CI, .64-1.64] for S. aureus). Prevalence of azithromycin-resistant S. pneumoniae was similar in both arms (1.8% vs 0.9% in children from the azithromycin and placebo arms, respectively; OR, 2.10 [95% CI, .30-23.38]); resistance to other antibiotics was also similar between arms. For S. aureus, there was no difference in azithromycin resistance between children in the azithromycin (3.1%) and placebo (2.6%) arms (OR, 1.22 [95% CI, .35-4.47]) or resistance to any other antibiotics.
Conclusions: The higher prevalence of S. aureus azithromycin resistance observed among women treated during labor and their babies 4 weeks after treatment had waned 12 months after delivery. Azithromycin intervention did not induce other antibiotic resistance to S. pneumoniae or S. aureus.
Clinical trials registration: NCT01800942.
Figures

References
-
- Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 2014; 143:225–45. - PubMed
-
- Bignell C, Garley J. Azithromycin in the treatment of infection with Neisseria gonorrhoeae. Sex Transm Infect 2010; 86:422–6. - PubMed
-
- Gebre T, Ayele B, Zerihun M et al. . Comparison of annual versus twice-yearly mass azithromycin treatment for hyperendemic trachoma in Ethiopia: a cluster-randomised trial. Lancet 2012; 379:143–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

