Trophoblast retrieval and isolation from the cervix: origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies
- PMID: 29608700
- PMCID: PMC6016716
- DOI: 10.1093/humupd/dmy008
Trophoblast retrieval and isolation from the cervix: origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies
Abstract
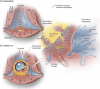
Background: Early during human development, the trophoblast lineage differentiates to commence placentation. Where the placenta contacts the uterine decidua, extravillous trophoblast (EVT) cells differentiate and invade maternal tissues. EVT cells, identified by expression of HLA-G, invade into uterine blood vessels (endovascular EVT), as well as glands (endoglandular EVT), and open such luminal structures towards the intervillous space of the placenta. Endoglandular invasion diverts the contents of uterine glands to the intervillous space, while glands near the margin of the placenta that also contain endoglandular EVT cells open into the reproductive tract. Cells of the trophoblast lineage have thus been recovered from the uterine cavity and endocervical canal. An emerging non-invasive technology [trophoblast retrieval and isolation from the cervix (TRIC)] isolates and examines EVT cells residing in the cervix to explore their origin, biology and relationship to pregnancy and fetal status.
Objective and rationale: This review explores the origins and possible uses of trophoblast cells obtained during ongoing pregnancies (weeks 5-20) by TRIC. We hypothesize that endoglandular EVT cells at the margins of the expanding placenta enter the uterine cavity and are carried together with uterine secretion products to the cervix where they can be retrieved from a Papanicolaou (Pap) smear. The advantages of TRIC for investigation of human placentation and prenatal testing will be considered. Evidence from the literature, and from archived in utero placental histological sections, is presented to support these hypotheses.
Search methods: We used 52 out of 80 publications that appeared between 1966 and 2017 and were found by searching the PubMed and Google Scholar databases. The studies described trophoblast invasion of uterine vessels and glands, as well as trophoblast cells residing in the reproductive tract. This was supplemented with literature on human placental health and disease.
Outcomes: The literature describes a variety of invasive routes taken by EVT cells at the fetal-maternal interface that could displace them into the reproductive tract. Since the 1970s, investigators have attempted to recover trophoblast cells from the uterus or cervix for prenatal diagnostics. Trophoblast cells from Pap smears obtained at 5-20 weeks of gestation have been purified (>95% β-hCG positive) by immunomagnetic isolation with nanoparticles linked to anti-HLA-G (TRIC). The isolated cells contain the fetal genome, and have an EVT-like expression profile. Similar EVT-like cells appear in the lumen of uterine glands and can be observed entering the uterine cavity along the margins of the placenta, suggesting that they are the primary source of cervical trophoblast cells. Cells isolated by TRIC can be used to accurately genotype the embryo/fetus by targeted next-generation sequencing. Biomarker protein expression quantified in cervical trophoblast cells after TRIC correlates with subsequent pregnancy loss, pre-eclampsia and fetal growth restriction. A key remaining question is the degree to which EVT cells in the cervix might differ from those in the basal plate and placental bed.
Wider implications: TRIC could one day provide a method of risk assessment for maternal and fetal disease, and reveal molecular pathways disrupted during the first trimester in EVT cells associated with placental maldevelopment. As perinatal interventions emerge for pregnancy disorders and inherited congenital disorders, TRIC could provide a key diagnostic tool for personalized precision medicine in obstetrics.
Figures



Similar articles
-
Endocervical trophoblast for interrogating the fetal genome and assessing pregnancy health at five weeks.Eur J Med Genet. 2019 Aug;62(8):103690. doi: 10.1016/j.ejmg.2019.103690. Epub 2019 Jun 18. Eur J Med Genet. 2019. PMID: 31226440 Free PMC article.
-
Quo vadis, trophoblast? Exploring the new ways of an old cell lineage.Placenta. 2017 Dec;60 Suppl 1(Suppl 1):S27-S31. doi: 10.1016/j.placenta.2017.04.021. Epub 2017 Apr 26. Placenta. 2017. PMID: 28483162 Free PMC article.
-
Evidence from the very beginning: endoglandular trophoblasts penetrate and replace uterine glands in situ and in vitro.Hum Reprod. 2015 Dec;30(12):2747-57. doi: 10.1093/humrep/dev266. Epub 2015 Oct 22. Hum Reprod. 2015. PMID: 26493408 Free PMC article.
-
Investigation of human trophoblast invasion in vitro.Hum Reprod Update. 2020 Jun 18;26(4):501-513. doi: 10.1093/humupd/dmaa017. Hum Reprod Update. 2020. PMID: 32441309 Free PMC article. Review.
-
Human trophoblast invasion: new and unexpected routes and functions.Histochem Cell Biol. 2018 Oct;150(4):361-370. doi: 10.1007/s00418-018-1699-0. Epub 2018 Jul 26. Histochem Cell Biol. 2018. PMID: 30046889 Free PMC article. Review.
Cited by
-
Noninvasive Prenatal Diagnostics: Recent Developments Using Circulating Fetal Nucleated Cells.Curr Obstet Gynecol Rep. 2019 Mar;8(1):1-8. Epub 2019 Jan 21. Curr Obstet Gynecol Rep. 2019. PMID: 31565541 Free PMC article.
-
Early human trophoblast development: from morphology to function.Cell Mol Life Sci. 2022 Jun 5;79(6):345. doi: 10.1007/s00018-022-04377-0. Cell Mol Life Sci. 2022. PMID: 35661923 Free PMC article. Review.
-
Enrichment of Placental Trophoblast Cells from Clinical Cervical Samples Using Differences in Surface Adhesion on an Inclined Plane.Ann Biomed Eng. 2021 Sep;49(9):2214-2227. doi: 10.1007/s10439-021-02742-x. Epub 2021 Mar 8. Ann Biomed Eng. 2021. PMID: 33686620
-
Specific innate immune cells uptake fetal antigen and display homeostatic phenotypes in the maternal circulation.J Leukoc Biol. 2022 Mar;111(3):519-538. doi: 10.1002/JLB.5HI0321-179RR. Epub 2021 Dec 10. J Leukoc Biol. 2022. PMID: 34889468 Free PMC article.
-
Endocervical trophoblast for interrogating the fetal genome and assessing pregnancy health at five weeks.Eur J Med Genet. 2019 Aug;62(8):103690. doi: 10.1016/j.ejmg.2019.103690. Epub 2019 Jun 18. Eur J Med Genet. 2019. PMID: 31226440 Free PMC article.
References
-
- ACOG Committee Opinion No. 638: first-trimester risk assessment for early-onset preeclampsia. Obstet Gynecol 2015;126:e25–e27. - PubMed
-
- ACOG/SMFM Committee Opinion No. 640: cell-free DNA screening for fetal aneuploidy. Obstet Gynecol 2015;126:e31–e37. - PubMed
-
- Adinolfi M, Davies A, Sharif S, Soothill P, Rodeck C.. Detection of trisomy 18 and Y-derived sequences in fetal nucleated cells obtained by transcervical flushing. Lancet 1993;342:403–404. - PubMed
-
- Adinolfi M, el-Hashemite N, Sherlock J, Ward RH, Petrou M, Rodeck C.. Prenatal detection of Hb mutations using transcervical cells. Prenat Diagn 1997;17:539–543. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

