Ancient Evolution of Mammarenaviruses: Adaptation via Changes in the L Protein and No Evidence for Host-Virus Codivergence
- PMID: 29608723
- PMCID: PMC5863214
- DOI: 10.1093/gbe/evy050
Ancient Evolution of Mammarenaviruses: Adaptation via Changes in the L Protein and No Evidence for Host-Virus Codivergence
Abstract
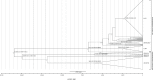
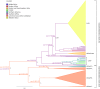
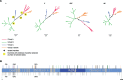
The Mammarenavirus genus includes several pathogenic species of rodent-borne viruses. Old World (OW) mammarenaviruses infect rodents in the Murinae subfamily and are mainly transmitted in Africa and Asia; New World (NW) mammarenaviruses are found in rodents of the Cricetidae subfamily in the Americas. We applied a selection-informed method to estimate that OW and NW mammarenaviruses diverged less than ∼45,000 years ago (ya). By incorporating phylogeographic inference, we show that NW mammarenaviruses emerged in the Latin America-Caribbean region ∼41,400-3,300 ya, whereas OW mammarenaviruses originated ∼23,100-1,880 ya, most likely in Southern Africa. Cophylogenetic analysis indicated that cospeciation did not contribute significantly to mammarenavirus-host associations. Finally, we show that extremely strong selective pressure on the viral polymerase accompanied the speciation of NW viruses. These data suggest that the evolutionary history of mammarenaviruses was not driven by codivergence with their hosts. The viral polymerase should be regarded as a major determinant of mammarenavirus adaptation.
Figures

Similar articles
-
Subspecific rodent taxa as the relevant host taxonomic level for mammarenavirus host specificity.Virology. 2023 Apr;581:116-127. doi: 10.1016/j.virol.2023.02.014. Epub 2023 Mar 8. Virology. 2023. PMID: 36958216
-
Host-Associated Distribution of Two Novel Mammarenaviruses in Rodents from Southern Africa.Viruses. 2022 Dec 29;15(1):99. doi: 10.3390/v15010099. Viruses. 2022. PMID: 36680139 Free PMC article.
-
Pseudotyped Viruses for Mammarenavirus.Adv Exp Med Biol. 2023;1407:279-297. doi: 10.1007/978-981-99-0113-5_15. Adv Exp Med Biol. 2023. PMID: 36920703
-
A Review of Mammarenaviruses and Rodent Reservoirs in the Americas.Ecohealth. 2022 Mar;19(1):22-39. doi: 10.1007/s10393-022-01580-0. Epub 2022 Mar 5. Ecohealth. 2022. PMID: 35247117 Free PMC article. Review.
-
Mammarenavirus Genetic Diversity and Its Biological Implications.Curr Top Microbiol Immunol. 2023;439:265-303. doi: 10.1007/978-3-031-15640-3_8. Curr Top Microbiol Immunol. 2023. PMID: 36592249 Review.
Cited by
-
Molecular characterization of a new highly divergent Mobala related arenavirus isolated from Praomys sp. rodents.Sci Rep. 2021 May 13;11(1):10188. doi: 10.1038/s41598-021-88046-5. Sci Rep. 2021. PMID: 33986310 Free PMC article.
-
A study on the codon usage bias of arenavirus common genes.Front Microbiol. 2025 Jan 23;15:1490076. doi: 10.3389/fmicb.2024.1490076. eCollection 2024. Front Microbiol. 2025. PMID: 39917269 Free PMC article.
-
The Biosafety Research Road Map: The Search for Evidence to Support Practices in the Laboratory-Crimean Congo Haemorrhagic Fever Virus and Lassa Virus.Appl Biosaf. 2023 Dec 1;28(4):216-229. doi: 10.1089/apb.2022.0044. Epub 2023 Dec 5. Appl Biosaf. 2023. PMID: 38090357 Free PMC article. Review.
-
Host phylogeny shapes viral transmission networks in an island ecosystem.Nat Ecol Evol. 2023 Nov;7(11):1834-1843. doi: 10.1038/s41559-023-02192-9. Epub 2023 Sep 7. Nat Ecol Evol. 2023. PMID: 37679456 Free PMC article.
-
Pathogenicity and virulence mechanisms of Lassa virus and its animal modeling, diagnostic, prophylactic, and therapeutic developments.Virulence. 2021 Dec;12(1):2989-3014. doi: 10.1080/21505594.2021.2000290. Virulence. 2021. PMID: 34747339 Free PMC article. Review.
References
-
- Abba Y. 2016. In vitro isolation and molecular identification of reptarenavirus in Malaysia. Virus Genes 52(5):640–650. - PubMed
-
- Beaujard P. 2007. East Africa, the Comoros Islands and Madagascar before the sixteenth century. Azania Archaeolog Res Afr 42(1):15–35.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

