Experiences of magnetic resonance imaging scanning in patients with pacemakers or implantable cardioverter-defibrillators
- PMID: 29609452
- PMCID: PMC6325447
- DOI: 10.3904/kjim.2017.251
Experiences of magnetic resonance imaging scanning in patients with pacemakers or implantable cardioverter-defibrillators
Abstract
Background/aims: Despite the U.S. Food and Drug Adminstration approving a magnetic resonance imaging (MRI)-conditional pacemaker system in 2011, many physicians remain reluctant to perform MRI scanning in patients with cardiac implantable electronic devices. Herein, we aimed to evaluate the real-world safety of MRI in these patients.
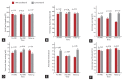
Methods: This single-center retrospective study examined the interrogation data and outcomes of patients with pacemakers or implantable cardioverter defibrillators who underwent MRI. MRI interrogation data were collected pre- and post-MRI and after 1 month of follow-up; these included the lead impedance, measured P- and R-wave amplitudes, and capture threshold. We compared these results between the magnetic resonance (MR)-conditional and conventional groups.
Results: From September 2013 to December 2015, 35 patients with cardiac implantable electronic devices underwent 43 MRI scans, with a mean follow-up of 5 months. Among these 35 patients, 14 (40%) had MR-conditional devices and 21 (60%) had conventional devices. Seven patients had high voltage devices, which were all the conventional type. There were no adverse events associated with MRI during the follow-up period, and there were no significant differences in the interrogation data changes between the conventional and MR-conditional groups.
Conclusion: This single-center retrospective study found that MRI can be performed safely in patients with pacemakers or implantable cardioverter defibrillators, regardless of the MRI support, as long as appropriate precautions are taken.
Keywords: Defibrillators, implantable; Interrogation data; Magnetic resonance imaging; Pacemakers; Safety.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

References
-
- Quint DJ. Indications for emergent MRI of the central nervous system. JAMA. 2000;283:853–855. - PubMed
-
- Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol. 2005;28:326–328. - PubMed
-
- Hayes DL, Holmes DR, Jr, Gray JE. Effect of 1.5 tesla nuclear magnetic resonance imaging scanner on implanted permanent pacemakers. J Am Coll Cardiol. 1987;10:782–786. - PubMed
-
- Shellock FG, Tkach JA, Ruggieri PM, Masaryk TJ. Cardiac pacemakers, ICDs, and loop recorder: evaluation of translational attraction using conventional (“long-bore”) and “short-bore” 1.5- and 3.0-Tesla MR systems. J Cardiovasc Magn Reson. 2003;5:387–397. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
