Extended-release oral treprostinil in the management of pulmonary arterial hypertension: clinical evidence and experience
- PMID: 29609511
- PMCID: PMC5941659
- DOI: 10.1177/1753466618766490
Extended-release oral treprostinil in the management of pulmonary arterial hypertension: clinical evidence and experience
Abstract
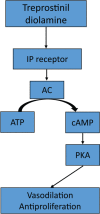
Treprostinil diolamine is the first oral prostacyclin approved for the treatment of pulmonary arterial hypertension (PAH) to improve exercise capacity. Clinical studies have demonstrated modest benefit as monotherapy, whereas no difference in exercise capacity was observed with combination therapy. However, these trials were limited by subtherapeutic dosing owing to intolerable adverse effects. Prostacyclin-related adverse effects, such as nausea, diarrhea, headache, flushing, and jaw pain, are prevalent. More recent pharmacokinetic and clinical studies illustrate the dose-response relationship and the importance of achieving clinically effective doses. Therefore, efforts to improve tolerability are paramount. Oral treprostinil is recommended to be administered three times daily in order to facilitate more rapid titration, higher doses achieved, and improved tolerability. Oral treprostinil has also been studied in carefully selected, stable patients that transitioned from parenteral or inhaled therapy with close monitoring for late deterioration. Ongoing clinical trials will determine the long-term effects of higher doses of oral treprostinil on clinical outcomes. This review describes the clinical evidence and practical experience with the use of oral treprostinil for PAH.
Keywords: oral treprostinil; prostacyclins; pulmonary arterial hypertension; pulmonary hypertension; treprostinil diolamine.
Conflict of interest statement
Figures
References
-
- Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122: 156–163. - PubMed
-
- Waxman AB. Oral prostacyclin therapy for pulmonary arterial hypertension: another step forward. Circulation 2013; 127: 563–565. - PubMed
-
- Jing ZC, Parikh K, Pulido T, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 2013; 127: 624–633. - PubMed
-
- Tapson VF, Torres F, Kermeen F, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest 2012; 142: 1383–1390. - PubMed
-
- Tapson VF, Jing ZC, Xu KF, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest 2013; 144: 952–958. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases