Metformin associated lactic acidosis: a case series of 28 patients treated with sustained low-efficiency dialysis (SLED) and long-term follow-up
- PMID: 29609531
- PMCID: PMC5879547
- DOI: 10.1186/s12882-018-0875-8
Metformin associated lactic acidosis: a case series of 28 patients treated with sustained low-efficiency dialysis (SLED) and long-term follow-up
Abstract
Background: Metformin associated lactic acidosis (MALA) is a well-known serious side effect of biguanides. However, the best treatment strategy remains a matter of debate. In the last 14 years, we observed a significant increase in hospitalizations for MALA to our Center. We report the outcomes of our clinical and therapeutic approach.
Methods: This is a single-center case series. Twenty-eight patients affected with MALA and acute kidney failure admitted between January 2000 and September 2014 were included. We analyzed comorbidities, laboratory tests and clinical parameters at admission, at 36 h and at discharge. All patients were treated with sustained low-efficiency dialysis (SLED) until normalization of serum lactate (≤ 3 mmol/L), bicarbonate (between 20 and 25 mmol/L) and potassium (between 4.0 and 5.1 mmol/L).
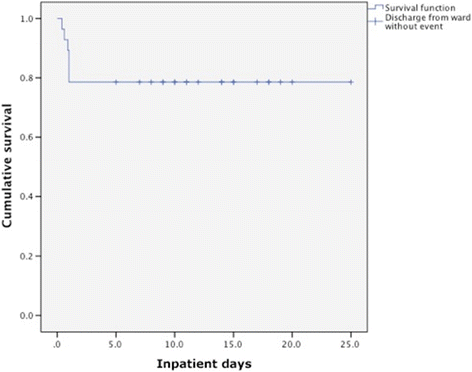
Results: The mortality rate was 21.4%, with all of the events occurring within 24 h from admission, and before or during the first hemodialysis treatment. Precipitating causes included; acute dehydration (86.4%), systemic inflammatory response syndrome (SIRS) (57.1%), sepsis (10.7%), nephrolithiasis (14.6%) and exposure to iodinated contrast (7.1%). No further episodes of lactic acidosis were described after discontinuing the drug over a mean follow-up of 27.2 months. Furthermore, while in 2010, we had a peak incidence of MALA of 76.8 cases per 100,000 patients on metformin, this rate fell after an education campaign conducted by specialists on the proper usage of metformin in patients at risk of MALA. Although the fall in incidence after the educational program was not necessarily causal, in 2014 the incidence was 32.9/100,000.
Conclusions: We report an improved mortality rate in patients affected with MALA and acute kidney injury treated with SLED compared with other series published in literature. Rapid introduction of effective hemodialysis is critical in improving outcomes.
Keywords: Acute kidney injury; Hemodialysis; Lactic acidosis; Metformin; SLED.
Conflict of interest statement
Ethics approval and consent to participate
The current study has been approved by the ethics committee of Azienda Ospedaliera G. Brotzu; at the admission, we asked for a written consent to every patient (or their relatives when patients were not able to give a consent). This retrospective research involved human subjects and human data. The dataset has been built in an anonymous way by direct care physicians. Only direct care physicians had the access to this database. No other third persons were allowed to consult this database, although totally anonymized. This study has been performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. Dr. Antonello Pani is a member of the editorial board (Associate Editor) of this Journal.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Associazione Medici Diabetologi. Standard italiani per la cura del diabete mellito 2014 wwwstandarditalianiit 2014.
-
- National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes statistics. Bethesda (MD): US Department of Health and Human Services. NIH publication 2014(Report no.: 94-3822).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

