Association between industry payments and prescribing costly medications: an observational study using open payments and medicare part D data
- PMID: 29609611
- PMCID: PMC5880069
- DOI: 10.1186/s12913-018-3043-8
Association between industry payments and prescribing costly medications: an observational study using open payments and medicare part D data
Abstract
Background: While many new medications may offer advantages over existing drugs, some newer drugs are reformulations of existing products that provide little innovation or incremental benefit while driving up drug costs. Despite the lack of benefit of these medications, prescribers may be motivated by payments made by the pharmaceutical industry. The objective of the study was to determine the association between payments made to physicians by the pharmaceutical industry and prescriptions for certain selected costly brand name drugs.
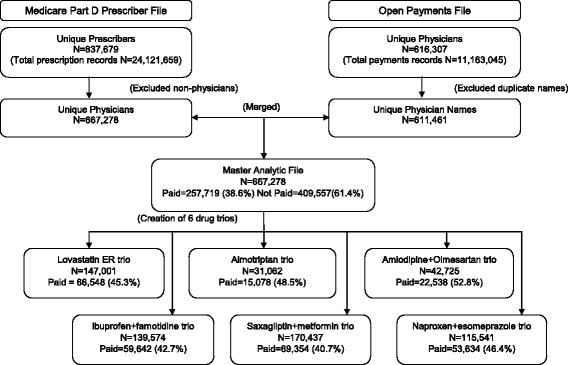
Methods: This was a cross-sectional, retrospective study linking the Open Payments Database and Medicare Part D Prescriber Public Use File for 2014, including 667,278 physicians who prescribed one of 6 brand-name drugs with less costly but similarly effective alternatives: lovastatin ER, almotriptan, amlodipine+olmesartan, ibuprofen+famotidine, saxagliptin+metformin and naproxen+esomeprazole. The primary outcome was the odds of a physician prescribing one of the selected drugs, and the primary predictor was the receipt of any payment from the pharmaceutical industry.
Results: The odds of prescribing 3 of the 6 drugs were increased among physicians who received industry payment, compared to those without payment: amlodipine+olmesartan, aOR 1.42, (95% CI 1.36-1.49); saxagliptin+metformin, aOR 1.50, (95% CI 1.42-1.59); and naproxen+esomeprazole, aOR 1.45, (95% CI 1.25-1.68). Payment from the manufacturer of the specific drug, compared to not receiving payment from the drug's manufacturer, was associated with increased odds of prescribing 4 of the 6 drugs: amlodipine+olmesartan, aOR 2.40, (95% CI 2.29-2.52), ibuprofen+famotidine, aOR 8.06, (95% CI 5.42-12.00), saxagliptin+metformin, aOR 2.21, (95% CI 2.10-2.34) and naproxen+esomeprazole, aOR 5.96, (95% CI 5.08-7.00).
Conclusions: A physician-industry financial relationship was associated with increased odds of prescribing costly brand-name drugs of uncertain medical benefit. Patients, as healthcare consumers, should demand transparency from their physicians about payment from the pharmaceutical industry to increase shared decision-making. Physician and policy makers need increased awareness and reflection on how industry payment influences their prescribing practices.
Keywords: Medicare Part D; Open Payments; Sunshine Act; physician-industry financial relationship.
Conflict of interest statement
Ethics approval and consent to participate
The Institutional Review Board (IRB) of the University of Houston determined the study to be exempt.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Organization of Economic Cooperation and Development. OECD (2015), Health at a Glance 2015: OECD Indicators. OECD Publishing, Paris. 10.1787/health_glance-2015-en.
-
- Chandon P. Innovative marketing strategies after patent expiry: The case of GSK's antibiotic Clamoxyl in France. International. Journal of Medical Marketing. 2004;4(1):65–73. doi: 10.1057/palgrave.jmm.5040144. - DOI
-
- Red Book Online. 2015. http://truvenhealth.com/Products/Micromedex/Product-Suites/Clinical-Know.... Accessed 7 Apr 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources