Use of analgesics in France, following dextropropoxyphene withdrawal
- PMID: 29609613
- PMCID: PMC5880096
- DOI: 10.1186/s12913-018-3058-1
Use of analgesics in France, following dextropropoxyphene withdrawal
Abstract
Background: In 2009, the European Medicines Agency recommended withdrawal of dextropropoxyphene (DXP); in March 2011 it was withdrawn from the market in France. Up until that time the combination dextropropoxyphene-paracetamol (DXP/PC) was widely used for analgesia. At withdrawal, French regulators recommended that DXP/PC be replaced by other step 2 analgesics, i.e. tramadol, codeine, or opium-containing drugs, or by PC for a weak level of pain. To investigate prescribing behaviours after DXP/PC withdrawal, dispensations of analgesics before and after withdrawal were analysed.
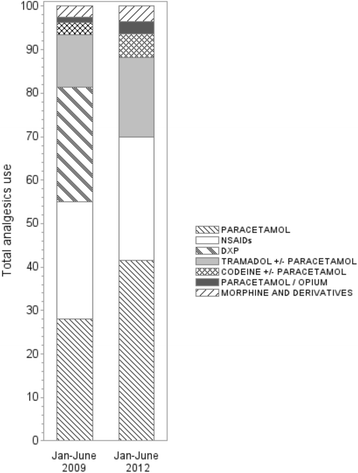
Methods: Aggregated dispensation data of analgesics prescribed between January 2009 and December 2012 in the Rhône-Alpes region were obtained from the general health insurance claims data; changes in analgesic dispensation over time were analysed with the ATC/DDD methodology. Pre (Jan-June 2009) and post-withdrawal (Jan-June 2012) changes of DDDs where computed for each analgesic step.
Results: The dispensations of DXP/PC experienced a two-step decrease until 2011. Over the withdrawal period 2009-2012, there was a 14% decrease in the overall use of analgesic (from 109 to 94 DDDs), while the use of step 2 analgesics declined by 46% (- 22 DDDs, from 47 to 25 DDDs). This latter decline included a cessation of use of DXP/PC (29 DDDs in 2009) that were only in part (+ 7 DDDs, from 18 to 25 DDDs) compensated by increased use of codeine, tramadol and opium, in monotherapy or combined with PC. For step 1 analgesics, use increased with 9%, mostly PC (+ 8 DDDs, from 31 to 39 DDDs). Step 3 analgesics dispensations remained largely unchanged over this period (around 3 DDDs).
Conclusions: In the Rhône-Alpes region, DXP/PC withdrawal was accompanied in part by an increased use of same level analgesics, and in part by an increased use of PC in monotherapy. The extent of DXP/PC use before withdrawal, and the increased use of PC after DXP withdrawal, underline the complexity of pain management.
Keywords: Analgesics; Drug withdrawal; Pain management; Real-life use.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable as this study was based on anonymized claims data.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- European Medicines Agency: European Medicines Agency recommends withdrawal of dextropropoxyphene-containing medicines (25/06/2009).2009 http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2.... .
-
- Retrait des médicaments contenant l’association dextropropoxyphène/paracétamol (Di-Antalvic® et ses génériques) ou dextropropoxyphène/paracétamol/caféine (Propofan® et ses génériques) [Internet]. [cited 2016 May 16]. Available from: http://ansm.sante.fr/var/ansm_site/storage/original/application/19ca2c73...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials