Sulforaphane attenuates pulmonary fibrosis by inhibiting the epithelial-mesenchymal transition
- PMID: 29609658
- PMCID: PMC5879815
- DOI: 10.1186/s40360-018-0204-7
Sulforaphane attenuates pulmonary fibrosis by inhibiting the epithelial-mesenchymal transition
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is a progressive and fatal disease with no effective treatment. The epithelial-mesenchymal transition (EMT) is a critical stage during the development of fibrosis. To assess the effect of sulforaphane (SFN) on the EMT and fibrosis using an in vitro transforming growth factor (TGF)-β1-induced model and an in vivo bleomycin (BLM)-induced model.
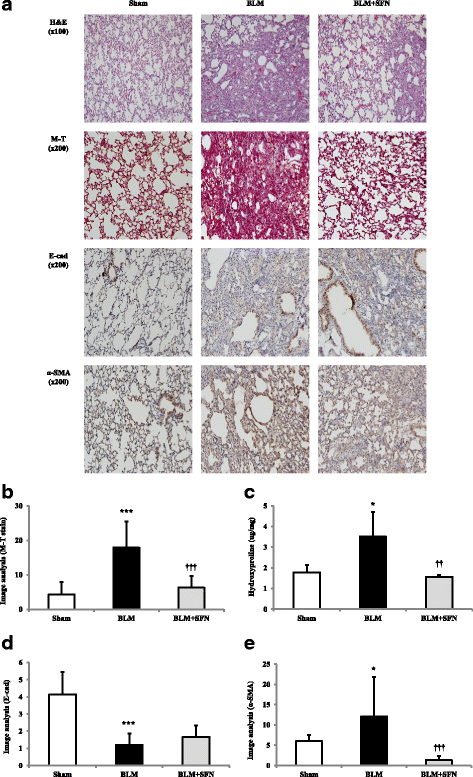
Methods: In vitro studies, cell viability, and cytotoxicity were measured using a Cell Counting Kit-8. The functional TGF-β1-induced EMT and fibrosis were assessed using western blotting and a quantitative real-time polymerase chain reaction. The lungs were analyzed histopathologically in vivo using hematoxylin and eosin and Masson's trichrome staining. The BLM-induced fibrosis was characterized by western blotting and immunohistochemical analyses for fibronectin, TGF-β1, E-cadherin (E-cad), and α-smooth muscle actin (SMA) in lung tissues.
Results: SFN reversed mesenchymal-like changes induced by TGF-β1 and restored cells to their epithelial-like morphology. The results confirmed that the expression of the epithelial marker, E-cadherin, increased after SFN treatment, while expression of the mesenchymal markers, N-cadherin, vimentin, and α-SMA decreased in A549 cells after SFN treatment. In addition, SFN inhibited TGF-β1-induced mRNA expression of the EMT-related transcription factors, Slug, Snail, and Twist. The SFN treatment attenuated TGF-β1-induced expression of fibrosis-related proteins, such as fibronection, collagen I, collagen IV, and α-SMA in MRC-5 cells. Furthermore, SFN reduced the TGF-β1-induced phosphorylation of SMAD2/3 protein in A549 cells and MRC-5 cells. BLM induced fibrosis in mouse lungs that was also attenuated by SFN treatment, and SFN treatment decreased BLM-induced fibronectin expression, TGF-β1 expression, and the levels of collagen I in the lungs of mice.
Conclusions: SFN showed a significant anti-fibrotic effect in TGF-β-treated cell lines and BLM-induced fibrosis in mice. These findings showed that SFN has anti-fibrotic activity that may be considered in the treatment of IPF.
Keywords: Bleomycin; Epithelial-mesenchymal transition; Idiopathic pulmonary fibrosis; Sulforaphane.
Conflict of interest statement
Ethics approval
The animal study in this research was approved by the Panel on Laboratory Animal Care of Gachon University [GIACUCR-011].
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Hydrogen inhalation attenuated bleomycin-induced pulmonary fibrosis by inhibiting transforming growth factor-β1 and relevant oxidative stress and epithelial-to-mesenchymal transition.Exp Physiol. 2019 Dec;104(12):1942-1951. doi: 10.1113/EP088028. Epub 2019 Oct 23. Exp Physiol. 2019. PMID: 31535412
-
Mushroom Inonotus sanghuang alleviates experimental pulmonary fibrosis: Implications for therapy of pulmonary fibrosis.Biomed Pharmacother. 2021 Jan;133:110919. doi: 10.1016/j.biopha.2020.110919. Epub 2020 Nov 14. Biomed Pharmacother. 2021. PMID: 33202282
-
Paeoniflorin suppresses TGF-β mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway.Acta Pharmacol Sin. 2016 Jun;37(6):794-804. doi: 10.1038/aps.2016.36. Epub 2016 May 2. Acta Pharmacol Sin. 2016. PMID: 27133302 Free PMC article.
-
[Research progress of anti-fibrotic drugs that inhibit epithelial-mesenchymal transition in pulmonary fibrosis].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2023 Jan 20;41(1):72-77. doi: 10.3760/cma.j.cn121094-20210628-00308. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2023. PMID: 36725301 Review. Chinese.
-
Cytokines as drivers: Unraveling the mechanisms of epithelial-mesenchymal transition in COVID-19 lung fibrosis.Biochem Biophys Res Commun. 2023 Dec 17;686:149118. doi: 10.1016/j.bbrc.2023.10.050. Epub 2023 Oct 14. Biochem Biophys Res Commun. 2023. PMID: 37931361 Review.
Cited by
-
A nutraceutical strategy for downregulating TGFβ signalling: prospects for prevention of fibrotic disorders, including post-COVID-19 pulmonary fibrosis.Open Heart. 2021 Apr;8(1):e001663. doi: 10.1136/openhrt-2021-001663. Open Heart. 2021. PMID: 33879509 Free PMC article. No abstract available.
-
Lung resident mesenchymal cells isolated from patients with the Bronchiolitis Obliterans Syndrome display a deregulated epigenetic profile.Sci Rep. 2018 Jul 24;8(1):11167. doi: 10.1038/s41598-018-29504-5. Sci Rep. 2018. PMID: 30042393 Free PMC article.
-
Investigation of miR-26b and miR-27b expressions and the effect of quercetin on fibrosis in experimental pulmonary fibrosis.J Mol Histol. 2024 Feb;55(1):25-35. doi: 10.1007/s10735-023-10168-z. Epub 2023 Oct 19. J Mol Histol. 2024. PMID: 37857923
-
Mechanism of miR-204-5p in exosomes derived from bronchoalveolar lavage fluid on the progression of pulmonary fibrosis via AP1S2.Ann Transl Med. 2021 Jul;9(13):1068. doi: 10.21037/atm-20-8033. Ann Transl Med. 2021. PMID: 34422980 Free PMC article.
-
Recent Advances (2015-2020) in Drug Discovery for Attenuation of Pulmonary Fibrosis and COPD.Molecules. 2023 Apr 24;28(9):3674. doi: 10.3390/molecules28093674. Molecules. 2023. PMID: 37175084 Free PMC article. Review.
References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

