Neonatal Transitions in Social Behavior and Their Implications for Autism
- PMID: 29609895
- PMCID: PMC6554740
- DOI: 10.1016/j.tics.2018.02.012
Neonatal Transitions in Social Behavior and Their Implications for Autism
Abstract
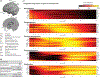
Within the context of early infant-caregiver interaction, we review a series of pivotal transitions that occur within the first 6 months of typical infancy, with emphasis on behavior and brain mechanisms involved in preferential orientation towards, and interaction with, other people. Our goal in reviewing these transitions is to better understand how they may lay a necessary and/or sufficient groundwork for subsequent phases of development, and also to understand how the breakdown thereof, when development is atypical and those transitions become derailed, may instead yield disability. We review these developmental processes in light of recent studies documenting disruptions to early-emerging brain and behavior mechanisms in infants later diagnosed with autism spectrum disorder, shedding light on the brain-behavior pathogenesis of autism.
Keywords: autism pathogenesis; infant learning; infant–caregiver adaptation; neonatal transitions; neurodevelopmental transitions; newborn predispositions.
Copyright © 2018. Published by Elsevier Ltd.
Figures



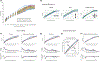
References
-
- Nagy E (2011) The newborn infant: a missing stage in developmental psychology. Infant Child Dev. 20, 3–19
-
- Wolff PH and Ferber R (1979) The development of behavior in human infants, premature and newborn. Annu. Rev. Neurosci. 2, 291–307 - PubMed
-
- Lieberman P (1985) The physiology of cry and speech in relation to linguistic behavior In Infant Crying (Lester BM, ed.), pp. 29–57, Plenum Press
-
- Ingram TTS (1962) Clinical significance of the infantile feeding reflexes. Dev. Med. Child Neurol. 4, 159–169
-
- Delaney AL and Arvedson JC (2008) Development of swallowing and feeding: prenatal through first year of life. Dev. Disabil. Res. Rev. 14, 105–117 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

