Discovery of a quinoline-based phenyl sulfone derivative as an antitrypanosomal agent
- PMID: 29609908
- PMCID: PMC5912169
- DOI: 10.1016/j.bmcl.2018.03.039
Discovery of a quinoline-based phenyl sulfone derivative as an antitrypanosomal agent
Abstract
A series of natural products-based phenyl sulfone derivative and their property-based analogues were investigated as potential growth inhibitors of Trypanosoma brucei. Trypanosoma brucei is a kinetoplastid protozoan parasite that causes trypanosomiasis. In this work, we found that nopol- and quinoline-based phenyl sulfone derivative were the most active and selective for T. brucei, and they were not reactive towards the active thiol of T. brucei's cysteine protease rhodesain. A thiol reactive variant of the quinoline-based phenyl sulfone was subsequently investigated and found to be a moderate inhibitor of rhodesain. The quinoline-based compound that is not reactive towards rhodesain can serve a template for phenotypic-based lead discovery while its thiol-active congener can serve as template for structure-based investigation of new antitrypanosomal agents.
Keywords: Cysteine protease; Natural products; Quinoline; Trypanosoma brucei.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
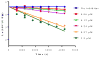
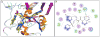


Similar articles
-
Vinyl sulfone-based inhibitors of trypanosomal cysteine protease rhodesain with improved antitrypanosomal activities.Bioorg Med Chem Lett. 2020 Jul 15;30(14):127217. doi: 10.1016/j.bmcl.2020.127217. Epub 2020 Apr 28. Bioorg Med Chem Lett. 2020. PMID: 32527539 Free PMC article.
-
Identification of non-peptidic cysteine reactive fragments as inhibitors of cysteine protease rhodesain.Bioorg Med Chem Lett. 2015 Oct 15;25(20):4509-12. doi: 10.1016/j.bmcl.2015.08.074. Epub 2015 Sep 2. Bioorg Med Chem Lett. 2015. PMID: 26342866 Free PMC article.
-
Novel 4-[4-(4-methylpiperazin-1-yl)phenyl]-6-arylpyrimidine derivatives and their antitrypanosomal activities against T.brucei.Bioorg Med Chem Lett. 2024 Sep 1;109:129825. doi: 10.1016/j.bmcl.2024.129825. Epub 2024 May 31. Bioorg Med Chem Lett. 2024. PMID: 38823730
-
The Inhibition of Cysteine Proteases Rhodesain and TbCatB: A Valuable Approach to Treat Human African Trypanosomiasis.Mini Rev Med Chem. 2016;16(17):1374-1391. doi: 10.2174/1389557515666160509125243. Mini Rev Med Chem. 2016. PMID: 27156518 Review.
-
Trypanocidal activity of marine natural products.Mar Drugs. 2013 Oct 22;11(10):4058-82. doi: 10.3390/md11104058. Mar Drugs. 2013. PMID: 24152565 Free PMC article. Review.
Cited by
-
Two Tags in One Probe: Combining Fluorescence- and Biotin-based Detection of the Trypanosomal Cysteine Protease Rhodesain.Chemistry. 2022 Nov 7;28(62):e202201636. doi: 10.1002/chem.202201636. Epub 2022 Sep 7. Chemistry. 2022. PMID: 35852812 Free PMC article.
-
A simple and convenient synthesis of 3-salicyloylquinoline-4-carboxylic esters from chromone and isatin.RSC Adv. 2019 Nov 12;9(63):37057-37060. doi: 10.1039/c9ra08124k. eCollection 2019 Nov 11. RSC Adv. 2019. PMID: 35539051 Free PMC article.
-
New quinoline-based triazole hybrid analogs as effective inhibitors of α-amylase and α-glucosidase: Preparation, in vitro evaluation, and molecular docking along with in silico studies.Front Chem. 2022 Sep 15;10:995820. doi: 10.3389/fchem.2022.995820. eCollection 2022. Front Chem. 2022. PMID: 36186602 Free PMC article.
-
Vinyl sulfone-based inhibitors of trypanosomal cysteine protease rhodesain with improved antitrypanosomal activities.Bioorg Med Chem Lett. 2020 Jul 15;30(14):127217. doi: 10.1016/j.bmcl.2020.127217. Epub 2020 Apr 28. Bioorg Med Chem Lett. 2020. PMID: 32527539 Free PMC article.
-
Nopol-Based Quinoline Derivatives as Antiplasmodial Agents.Molecules. 2021 Feb 14;26(4):1008. doi: 10.3390/molecules26041008. Molecules. 2021. PMID: 33673007 Free PMC article.
References
-
- WHO. Third WHO Report on Neglected Tropical Diseases. World Health Organization; Geneva: 2015. Investing to Overcome the Global Impact of Neglected Tropical Diseases.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

