A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel
- PMID: 29610524
- PMCID: PMC5880808
- DOI: 10.1038/s41467-018-03570-9
A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel
Abstract
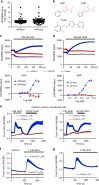
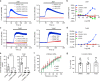
Piezo1 represents a prototype of eukaryotic mechanotransduction channels. The full-length 2547-residue mouse Piezo1 possesses a unique 38-transmembrane-helix (TM) topology and is organized into a three-bladed, propeller-shaped architecture, comprising a central ion-conducting pore, three peripheral blade-like structures, and three 90-Å-long intracellular beam-resembling structures that bridge the blades to the pore. However, how mechanical force and chemicals activate the gigantic Piezo1 machinery remains elusive. Here we identify a novel set of Piezo1 chemical activators, termed Jedi, which activates Piezo1 through the extracellular side of the blade instead of the C-terminal extracellular domain of the pore, indicating long-range allosteric gating. Remarkably, Jedi-induced activation of Piezo1 requires the key mechanotransduction components, including the two extracellular loops in the distal blade and the two leucine residues in the proximal end of the beam. Thus, Piezo1 employs the peripheral blade-beam-constituted lever-like apparatus as a designated transduction pathway for long-distance mechano- and chemical-gating of the pore.
Conflict of interest statement
The authors declare no competing interests.
Figures
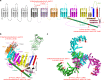



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

