Guidance and Self-Sorting of Active Swimmers: 3D Periodic Arrays Increase Persistence Length of Human Sperm Selecting for the Fittest
- PMID: 29610725
- PMCID: PMC5827459
- DOI: 10.1002/advs.201700531
Guidance and Self-Sorting of Active Swimmers: 3D Periodic Arrays Increase Persistence Length of Human Sperm Selecting for the Fittest
Abstract
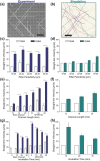
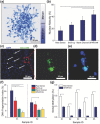
Male infertility is a reproductive disease, and existing clinical solutions for this condition often involve long and cumbersome sperm sorting methods, including preprocessing and centrifugation-based steps. These methods also fall short when sorting for sperm free of reactive oxygen species, DNA damage, and epigenetic aberrations. Although several microfluidic platforms exist, they suffer from structural complexities, i.e., pumps or chemoattractants, setting insurmountable barriers to clinical adoption. Inspired by the natural filter-like capabilities of the female reproductive tract for sperm selection, a model-driven design, featuring pillar arrays that efficiently and noninvasively isolate highly motile and morphologically normal sperm, with lower epigenetic global methylation, from raw semen, is presented. The Simple Periodic ARray for Trapping And isolatioN (SPARTAN) created here modulates the directional persistence of sperm, increasing the spatial separation between progressive and nonprogressive motile sperm populations within an unprecedentedly short 10 min assay time. With over 99% motility of sorted sperm, a 5-fold improvement in morphology, 3-fold increase in nuclear maturity, and 2-4-fold enhancement in DNA integrity, SPARTAN offers to standardize sperm selection while eliminating operator-to-operator variations, centrifugation, and flow. SPARTAN can also be applied in other areas, including conservation ecology, breeding of farm animals, and design of flagellar microrobots for diagnostics.
Keywords: fertility; multiparticle collision dynamics; multiscale simulations; persistence length; sperm sorting.
Figures



References
-
- Turchi P., in Clinical Management of Male Infertility (Eds: Giorgio C., Giovanni B.), Springer International Publishing; 2015, pp. 5–11.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
