Non-canonical regulation of glutathione and trehalose biosynthesis characterizes non- Saccharomyces wine yeasts with poor performance in active dry yeast production
- PMID: 29610760
- PMCID: PMC5878686
- DOI: 10.15698/mic2018.04.624
Non-canonical regulation of glutathione and trehalose biosynthesis characterizes non- Saccharomyces wine yeasts with poor performance in active dry yeast production
Abstract
Several yeast species, belonging to Saccharomyces and non-Saccharomyces genera, play fundamental roles during spontaneous must grape fermentation, and recent studies have shown that mixed fermentations, co-inoculated with S. cerevisiae and non-Saccharomyces strains, can improve wine organoleptic properties. During active dry yeast (ADY) production, antioxidant systems play an essential role in yeast survival and vitality as both biomass propagation and dehydration cause cellular oxidative stress and negatively affect technological performance. Mechanisms for adaptation and resistance to desiccation have been described for S. cerevisiae, but no data are available on the physiology and oxidative stress response of non-Saccharomyces wine yeasts and their potential impact on ADY production. In this study we analyzed the oxidative stress response in several non-Saccharomyces yeast species by measuring the activity of reactive oxygen species (ROS) scavenging enzymes, e.g., catalase and glutathione reductase, accumulation of protective metabolites, e.g., trehalose and reduced glutathione (GSH), and lipid and protein oxidation levels. Our data suggest that non-canonical regulation of glutathione and trehalose biosynthesis could cause poor fermentative performance after ADY production, as it corroborates the corrective effect of antioxidant treatments, during biomass propagation, with both pure chemicals and food-grade argan oil.
Keywords: active dry wine yeasts; antioxidant defense; food-grade argan oil; non-Saccharomyces yeasts; oxidative damage.
Conflict of interest statement
Conflict of interest: The authors declare no commercial or financial conflict of interest.
Figures
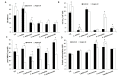



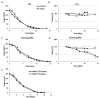
References
-
- Andorra I, Berradre M, Rozes N, Mas A, Guillamon JM, Esteve-Zarzoso B. Effect of pure and mixed cultures of the main wine yeast species on grape must fermentations. Eur Food Res Technol. 2010;231:215–224. doi: 10.1016/j.fm.2008.05.005. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
