3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial
- PMID: 29611518
- PMCID: PMC5883334
- DOI: 10.1016/S1470-2045(18)30093-7
3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial
Abstract
Background: 6 months of oxaliplatin-containing chemotherapy is usually given as adjuvant treatment for stage 3 colorectal cancer. We investigated whether 3 months of oxaliplatin-containing chemotherapy would be non-inferior to the usual 6 months of treatment.
Methods: The SCOT study was an international, randomised, phase 3, non-inferiority trial done at 244 centres. Patients aged 18 years or older with high-risk stage II and stage III colorectal cancer underwent central randomisation with minimisation for centre, choice of regimen, sex, disease site, N stage, T stage, and the starting dose of capecitabine. Patients were assigned (1:1) to receive 3 months or 6 months of adjuvant oxaliplatin-containing chemotherapy. The chemotherapy regimens could consist of CAPOX (capecitabine and oxaliplatin) or FOLFOX (bolus and infused fluorouracil with oxaliplatin). The regimen was selected before randomisation in accordance with choices of the patient and treating physician. The primary study endpoint was disease-free survival and the non-inferiority margin was a hazard ratio of 1·13. The primary analysis was done in the intention-to-treat population and safety was assessed in patients who started study treatment. This trial is registered with ISRCTN, number ISRCTN59757862, and follow-up is continuing.
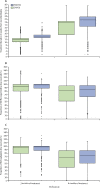
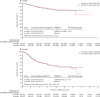
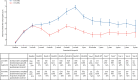
Findings: 6088 patients underwent randomisation between March 27, 2008, and Nov 29, 2013. The intended treatment was FOLFOX in 1981 patients and CAPOX in 4107 patients. 3044 patients were assigned to 3 month group and 3044 were assigned to 6 month group. Nine patients in the 3 month group and 14 patients in the 6 month group did not consent for their data to be used, leaving 3035 patients in the 3 month group and 3030 patients in the 6 month group for the intention-to-treat analyses. At the cutoff date for analysis, there had been 1482 disease-free survival events, with 740 in the 3 month group and 742 in the 6 month group. 3 year disease-free survival was 76·7% (95% CI 75·1-78·2) for the 3 month group and 77·1% (75·6-78·6) for the 6 month group, giving a hazard ratio of 1·006 (0·909-1·114, test for non-inferiority p=0·012), significantly below the non-inferiority margin. Peripheral neuropathy of grade 2 or worse was more common in the 6 month group (237 [58%] of 409 patients for the subset with safety data) than in the 3 month group (103 [25%] of 420) and was long-lasting and associated with worse quality of life. 1098 serious adverse events were reported (492 reports in the 3 month group and 606 reports in the 6 month group) and 32 treatment-related deaths occurred (16 in each group).
Interpretation: In the whole study population, 3 months of oxaliplatin-containing adjuvant chemotherapy was non-inferior to 6 months of the same therapy for patients with high-risk stage II and stage III colorectal cancer and was associated with reduced toxicity and improved quality of life. Despite the fact the study was underpowered, these data suggest that a shorter duration leads to similar survival outcomes with better quality of life and thus might represent a new standard of care.
Funding: Medical Research Council, Swedish Cancer Society, NETSCC, and Cancer Research UK.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures




Comment in
-
Adjuvant therapy in colon cancer: less is more.Lancet Oncol. 2018 Apr;19(4):442-443. doi: 10.1016/S1470-2045(18)30127-X. Lancet Oncol. 2018. PMID: 29611513 No abstract available.
References
-
- Ferlay J, Soerjomataram I, Ervik M. International Agency for Research on Cancer; Lyon: 2013. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC cancer base no. 11.
-
- Moertel CG, Fleming TR, Macdonald JS. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–358. - PubMed
-
- Andre T, Boni C, Mounedji-Boudiaf L. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. - PubMed
-
- Kuebler JP, Wieand HS, O'Connell MJ. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. - PubMed
-
- Haller DG, Tabernero J, Maroun J. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

