Non-Metastatic Cutaneous Melanoma Induces Chronodisruption in Central and Peripheral Circadian Clocks
- PMID: 29614021
- PMCID: PMC5979525
- DOI: 10.3390/ijms19041065
Non-Metastatic Cutaneous Melanoma Induces Chronodisruption in Central and Peripheral Circadian Clocks
Abstract
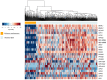
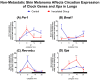
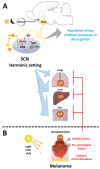
The biological clock has received increasing interest due to its key role in regulating body homeostasis in a time-dependent manner. Cancer development and progression has been linked to a disrupted molecular clock; however, in melanoma, the role of the biological clock is largely unknown. We investigated the effects of the tumor on its micro- (TME) and macro-environments (TMaE) in a non-metastatic melanoma model. C57BL/6J mice were inoculated with murine B16-F10 melanoma cells and 2 weeks later the animals were euthanized every 6 h during 24 h. The presence of a localized tumor significantly impaired the biological clock of tumor-adjacent skin and affected the oscillatory expression of genes involved in light- and thermo-reception, proliferation, melanogenesis, and DNA repair. The expression of tumor molecular clock was significantly reduced compared to healthy skin but still displayed an oscillatory profile. We were able to cluster the affected genes using a human database and distinguish between primary melanoma and healthy skin. The molecular clocks of lungs and liver (common sites of metastasis), and the suprachiasmatic nucleus (SCN) were significantly affected by tumor presence, leading to chronodisruption in each organ. Taken altogether, the presence of non-metastatic melanoma significantly impairs the organism's biological clocks. We suggest that the clock alterations found in TME and TMaE could impact development, progression, and metastasis of melanoma; thus, making the molecular clock an interesting pharmacological target.
Keywords: cancer; central and peripheral clocks; chronodisruption; melanoma; tumor macroenvironment; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

