Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: A prospective study in children
- PMID: 29614081
- PMCID: PMC5882115
- DOI: 10.1371/journal.pmed.1002548
Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: A prospective study in children
Abstract
Background: Around 0.3% of newborns will develop autoimmunity to pancreatic beta cells in childhood and subsequently develop type 1 diabetes before adulthood. Primary prevention of type 1 diabetes will require early intervention in genetically at-risk infants. The objective of this study was to determine to what extent genetic scores (two previous genetic scores and a merged genetic score) can improve the prediction of type 1 diabetes.
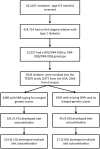
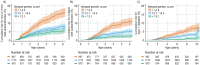
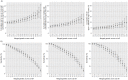
Methods and findings: The Environmental Determinants of Diabetes in the Young (TEDDY) study followed genetically at-risk children at 3- to 6-monthly intervals from birth for the development of islet autoantibodies and type 1 diabetes. Infants were enrolled between 1 September 2004 and 28 February 2010 and monitored until 31 May 2016. The risk (positive predictive value) for developing multiple islet autoantibodies (pre-symptomatic type 1 diabetes) and type 1 diabetes was determined in 4,543 children who had no first-degree relatives with type 1 diabetes and either a heterozygous HLA DR3 and DR4-DQ8 risk genotype or a homozygous DR4-DQ8 genotype, and in 3,498 of these children in whom genetic scores were calculated from 41 single nucleotide polymorphisms. In the children with the HLA risk genotypes, risk for developing multiple islet autoantibodies was 5.8% (95% CI 5.0%-6.6%) by age 6 years, and risk for diabetes by age 10 years was 3.7% (95% CI 3.0%-4.4%). Risk for developing multiple islet autoantibodies was 11.0% (95% CI 8.7%-13.3%) in children with a merged genetic score of >14.4 (upper quartile; n = 907) compared to 4.1% (95% CI 3.3%-4.9%, P < 0.001) in children with a genetic score of ≤14.4 (n = 2,591). Risk for developing diabetes by age 10 years was 7.6% (95% CI 5.3%-9.9%) in children with a merged score of >14.4 compared with 2.7% (95% CI 1.9%-3.6%) in children with a score of ≤14.4 (P < 0.001). Of 173 children with multiple islet autoantibodies by age 6 years and 107 children with diabetes by age 10 years, 82 (sensitivity, 47.4%; 95% CI 40.1%-54.8%) and 52 (sensitivity, 48.6%, 95% CI 39.3%-60.0%), respectively, had a score >14.4. Scores were higher in European versus US children (P = 0.003). In children with a merged score of >14.4, risk for multiple islet autoantibodies was similar and consistently >10% in Europe and in the US; risk was greater in males than in females (P = 0.01). Limitations of the study include that the genetic scores were originally developed from case-control studies of clinical diabetes in individuals of mainly European decent. It is, therefore, possible that it may not be suitable to all populations.
Conclusions: A type 1 diabetes genetic score identified infants without family history of type 1 diabetes who had a greater than 10% risk for pre-symptomatic type 1 diabetes, and a nearly 2-fold higher risk than children identified by high-risk HLA genotypes alone. This finding extends the possibilities for enrolling children into type 1 diabetes primary prevention trials.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: A patent has been applied for (EP17178396/LU100334) with the title "Method the risk to develop type 1 diabetes" by Helmholtz Zentrum München Deutsches Forschungszentrum für Gesundheit und Umwelt (GmbH). EB, AGZ, CW and JK are one of the inventors. The patent includes the genetic score that is examined in the manuscript. RAO has a personal funding from Diabetes UK to study the biology of Type 1 diabetes (this includes a research grant to work on genetic risk scores in Type 1 diabetes). RAO has a UK Medical Research Council confidence in concept grant to turn a type 1 diabetes genetic risk score into a diagnostic test for clinical practice. AB, MH, KV, MNW, ML, ATH, BIF, AKS, WAH, JPK, AL, MJR, JXS, JT, BA, SSR have declared that no other competing interests exist.
Figures


References
-
- Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–13. doi: 10.1056/NEJMoa1414850 - DOI - PMC - PubMed
-
- Krischer JP, Lynch KF, Schatz DA, Ilonen J, Lernmark Å, Hagopian WA, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58:980–7. doi: 10.1007/s00125-015-3514-y - DOI - PMC - PubMed
-
- Knip M, Åkerblom HK, Becker D, Dosch HM, Dupre J, Fraser W, et al. Hydrolyzed infant formula and early β-cell autoimmunity: a randomized clinical trial. JAMA. 2014;311:2279–87. doi: 10.1001/jama.2014.5610 - DOI - PMC - PubMed
-
- Bonifacio E, Ziegler AG, Klingensmith G, Schober E, Bingley PJ, Rottenkolber M, et al. Effects of high dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT randomized clinical trial. JAMA. 2015;313:1541–9. doi: 10.1001/jama.2015.2928 - DOI - PubMed
-
- Vriezinga SL, Auricchio R, Bravi E, Castillejo G, Chmielewska A, Crespo Escobar P, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med. 2014;371(14):1304–15. doi: 10.1056/NEJMoa1404172 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK063821/DK/NIDDK NIH HHS/United States
- UC4 DK063863/DK/NIDDK NIH HHS/United States
- MC_PC_15047/MRC_/Medical Research Council/United Kingdom
- HHSN267200700014C/DK/NIDDK NIH HHS/United States
- U01 DK063861/DK/NIDDK NIH HHS/United States
- UL1 TR001427/TR/NCATS NIH HHS/United States
- U01 DK063790/DK/NIDDK NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- U01 DK063836/DK/NIDDK NIH HHS/United States
- U01 DK063829/DK/NIDDK NIH HHS/United States
- U01 DK063865/DK/NIDDK NIH HHS/United States
- UC4 DK095300/DK/NIDDK NIH HHS/United States
- UC4 DK063861/DK/NIDDK NIH HHS/United States
- UC4 DK063829/DK/NIDDK NIH HHS/United States
- UC4 DK063821/DK/NIDDK NIH HHS/United States
- UC4 DK117483/DK/NIDDK NIH HHS/United States
- UC4 DK063836/DK/NIDDK NIH HHS/United States
- UC4 DK112243/DK/NIDDK NIH HHS/United States
- UC4 DK063865/DK/NIDDK NIH HHS/United States
- U01 DK063863/DK/NIDDK NIH HHS/United States
- UC4 DK106955/DK/NIDDK NIH HHS/United States
- UC4 DK100238/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

