Orally Available Soluble Epoxide Hydrolase/Phosphodiesterase 4 Dual Inhibitor Treats Inflammatory Pain
- PMID: 29614224
- PMCID: PMC5933862
- DOI: 10.1021/acs.jmedchem.7b01804
Orally Available Soluble Epoxide Hydrolase/Phosphodiesterase 4 Dual Inhibitor Treats Inflammatory Pain
Abstract
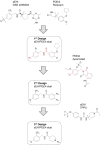
Inspired by previously discovered enhanced analgesic efficacy between soluble epoxide hydrolase (sEH) and phosphodiesterase 4 (PDE4) inhibitors, we designed, synthesized and characterized 21 novel sEH/PDE4 dual inhibitors. The best of these displayed good efficacy in in vitro assays. Further pharmacokinetic studies of a subset of four selected compounds led to the identification of a bioavailable dual inhibitor N-(4-methoxy-2-(trifluoromethyl)benzyl)-1-propionylpiperidine-4-carboxamide (MPPA). In a lipopolysaccharide induced inflammatory pain rat model, MPPA rapidly increased in the blood ( Tmax = 30 min; Cmax = 460 nM) after oral administration of 3 mg/kg and reduced inflammatory pain with rapid onset of action correlating with blood levels over a time course of 4 h. Additionally, MPPA does not alter self-motivated exploration of rats with inflammatory pain or the withdrawal latency in control rats.
Figures


References
-
- Ashton MJ, Cook DC, Fenton G, Karlsson JA, Palfreyman MN, Raeburn D, Ratcliffe AJ, Souness JE, Thurairatnam S, Vicker N. Selective Type IV Phosphodiesterase Inhibitors as Antiasthmatic Agents. The Syntheses and Biological Activities of 3-(Cyclopentyloxy)-4-Methoxybenzamides and Analogues. J Med Chem. 1994;37(11):1696–1703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

