Targeting AMPK Signaling as a Neuroprotective Strategy in Parkinson's Disease
- PMID: 29614701
- PMCID: PMC6004921
- DOI: 10.3233/JPD-171296
Targeting AMPK Signaling as a Neuroprotective Strategy in Parkinson's Disease
Abstract
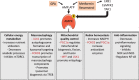
Parkinson's disease (PD) is the second most common neurodegenerative disorder. It is characterized by the accumulation of intracellular α-synuclein aggregates and the degeneration of nigrostriatal dopaminergic neurons. While no treatment strategy has been proven to slow or halt the progression of the disease, there is mounting evidence from preclinical PD models that activation of 5'-AMP-activated protein kinase (AMPK) may have broad neuroprotective effects. Numerous dietary supplements and pharmaceuticals (e.g., metformin) that increase AMPK activity are available for use in humans, but clinical studies of their effects in PD patients are limited. AMPK is an evolutionarily conserved serine/threonine kinase that is activated by falling energy levels and functions to restore cellular energy balance. However, in response to certain cellular stressors, AMPK activation may exacerbate neuronal atrophy and cell death. This review describes the regulation and functions of AMPK, evaluates the controversies in the field, and assesses the potential of targeting AMPK signaling as a neuroprotective treatment for PD.
Keywords: 5′-AMP-activated protein kinase; Parkinson’s disease; alpha-synuclein; autophagy; metformin; mitochondria; resveratrol.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

